Interactions between kinases and phosphatases in the rapid control of brain aromatase
- PMID: 16101893
- PMCID: PMC2040223
- DOI: 10.1111/j.1365-2826.2005.01344.x
Interactions between kinases and phosphatases in the rapid control of brain aromatase
Abstract
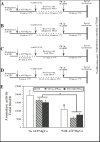
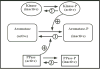
Aromatization of testosterone into oestradiol plays a key role in the activation of male sexual behaviour in many vertebrate species. Rapid changes in brain aromatase activity have recently been identified and the resulting changes in local oestrogen bioavailability could modulate fast behavioural responses to oestrogens. In quail hypothalamic homogenates, aromatase activity is down-regulated within minutes by calcium-dependent phosphorylations in the presence of ATP, MgCl2 and CaCl2 (ATP/Mg/Ca). Three kinases (protein kinases A and C and calmodulin kinase; PKA, PKC and CAMK) are potentially implicated in this process. If kinases decrease aromatase activity in a reversible manner, then it would be expected that the enzymatic activity would increase and/or return to baseline levels in the presence of phosphatases. We showed previously that 0.1 mM vanadate (a general inhibitor of protein phosphatases) significantly decreases aromatase activity but specific protein phosphatases that could up-regulate aromatase activity have not been identified to date. The reversibility of aromatase activity inhibition by phosphorylations was investigated in the present study using alkaline and acid phosphatase (Alk and Ac PPase). Unexpectedly, Alk PPase inhibited aromatase activity in a dose-dependent manner in the presence, as well as in the absence, of ATP/Mg/Ca. By contrast, Ac PPase completely blocked the inhibitory effects of ATP/Mg/Ca on aromatase activity, even if it moderately inhibited aromatase activity in the absence of ATP/Mg/Ca. However, the addition of Ac PPase was unable to restore aromatase activity after it had been inhibited by exposure to ATP/Mg/Ca. Taken together, these data suggest that, amongst the 15 potential consensus phosphorylation sites identified on the quail aromatase sequence, some must be constitutively phosphorylated for the enzyme to be active whereas phosphorylation of the others is involved in the rapid inhibition of aromatase activity by the competitive effects of protein kinases and phosphatases. Two out of these 15 putative phosphorylation sites occur in an environment corresponding to the consensus sites for PKC, PKA (and possibly a CAMK) and, in all probability, represent the sites whose phosphorylation rapidly blocks enzyme activity.
Figures

References
-
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. - PubMed
-
- Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. 1. Vol. 3. Springer Verlag; Berlin: 1989. pp. 105–159.
-
- Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. - PubMed
-
- Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. - PubMed
-
- Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

