Involvement of the p97-Ufd1-Npl4 complex in the regulated endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors
- PMID: 16103111
- PMCID: PMC1483127
- DOI: 10.1074/jbc.M508890200
Involvement of the p97-Ufd1-Npl4 complex in the regulated endoplasmic reticulum-associated degradation of inositol 1,4,5-trisphosphate receptors
Abstract
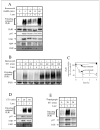
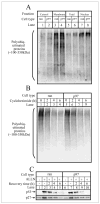
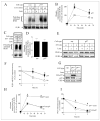
Inositol 1,4,5-trisphosphate (IP(3)) receptors form tetrameric, IP(3)-gated channels in endoplasmic reticulum membranes that govern the release of Ca(2+) from this organelle. In response to activation of certain G protein-coupled receptors that persistently elevate IP(3) concentration, IP(3) receptors are ubiquitinated and degraded by the ubiquitin-proteasome pathway. IP(3) receptor ubiquitination is mediated by the ubiquitin-conjugating enzyme, (mam)Ubc7, a component of the endoplasmic reticulum-associated degradation pathway. However, the mechanism by which ubiquitinated IP(3) receptors are transferred to the proteasome is not known. Here, we examine this process and show in several mammalian cell types that the ATPase p97 associates with IP(3) receptors in response to hormonal stimuli that induce IP(3) receptor ubiquitination. To examine the functional relevance of the p97 interaction with IP(3) receptors, we stably and specifically reduced p97 protein levels by 62 +/- 3% in Rat-1 fibroblasts using RNA interference. In these cells, endothelin-1-induced IP(3) receptor degradation was markedly retarded and the accumulation of ubiquitinated IP(3) receptors was markedly enhanced. These effects were reversed by expression of exogenous p97. In addition, Ufd1 and Npl4, which complex with p97, also associated with IP(3) receptors upon hormonal stimulation. We conclude that the p97-Ufd1-Npl4 complex couples ubiquitinated IP(3) receptors to proteasomal degradation and, thus, plays a key role in IP(3) receptor processing. These data also establish that the p97-Ufd1-Npl4 complex mediates endoplasmic reticulum-associated degradation in mammalian cells.
Figures

References
-
- Patterson RL, Boehning D, Snyder SH. Annu Rev Biochem. 2004;73:437–465. - PubMed
-
- Berridge MJ, Lipp P, Bootman MD. Nat Rev Mol Cell Biol. 2000;1:11–21. - PubMed
-
- Wojcikiewicz RJH. J Biol Chem. 1995;270:11678–11683. - PubMed
-
- Taylor CW, Genazzani AA, Morris SA. Cell Calcium. 1999;26:237–251. - PubMed
-
- Taylor CW, da Fonseca PCA, Morris EP. Trends Biochem Sci. 2004;29:210–219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

