Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein
- PMID: 16103160
- PMCID: PMC1193588
- DOI: 10.1128/JVI.79.17.11095-11104.2005
Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein
Abstract
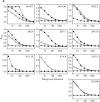
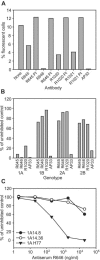
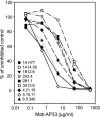
Hepatitis C virus (HCV) remains a significant threat to the general health of the world's population, and there is a pressing need for the development of new treatments and preventative vaccines. Here, we describe the generation of retrovirus-based pseudoparticles (HCVpp) incorporating a panel of full-length E1E2 clones representative of the major genotypes 1 through 6, and their application to assess the reactivity and neutralizing capability of antisera and monoclonal antibodies raised against portions of the HCV E2 envelope protein. Rabbit antisera raised against either the first hypervariable region or ectodomain of E2 showed limited and strain specific neutralization. By contrast, the monoclonal antibody (MAb) AP33 demonstrated potent neutralization of infectivity against HCVpp carrying E1E2 representative of all genotypes tested. The concentration of AP33 required to achieve 50% inhibition of infection by HCVpp of diverse genotypes ranged from 0.6 to 32 mug/ml. The epitope recognized by MAb AP33 is linear and highly conserved across different genotypes of HCV. Thus, identification of a broadly neutralizing antibody that recognizes a linear epitope is likely to be of significant benefit to future vaccine and therapeutic antibody development.
Figures

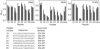

References
-
- Allander, T., K. Drakenberg, A. Beyene, D. Rosa, S. Abrignani, M. Houghton, A. Widell, L. Grillner, and M. A. A. Persson. 2000. Recombinant human monoclonal antibodies against different conformational epitopes of the E2 envelope glycoprotein of hepatitis C virus that inhibit its interaction with CD81. J. Gen. Virol. 81:2451-2459. - PubMed
-
- Alter, M. J., H. S. Margolis, K. Krawczynski, F. N. Judson, A. Mares, W. J. Alexander, H. Pin Ya, J. K. Miller, M. A. Gerber, R. E. Sampliner, E. L. Meeks, and M. J. Beach. 1992. The natural history of community-acquired hepatitis C in the United States. N. Engl. J. Med. 327:1899-1905. - PubMed
-
- Anonymous. 1999. Global surveillance and control of hepatitis C. J. Viral Hepat. 6:35-47. - PubMed
-
- Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

