Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia?
- PMID: 16103179
- PMCID: PMC1193583
- DOI: 10.1128/JVI.79.17.11269-11279.2005
Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia?
Abstract
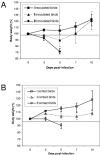
Wild waterfowl are the natural reservoir of all influenza A viruses, and these viruses are usually nonpathogenic in these birds. However, since late 2002, H5N1 outbreaks in Asia have resulted in mortality among waterfowl in recreational parks, domestic flocks, and wild migratory birds. The evolutionary stasis between influenza virus and its natural host may have been disrupted, prompting us to ask whether waterfowl are resistant to H5N1 influenza virus disease and whether they can still act as a reservoir for these viruses. To better understand the biology of H5N1 viruses in ducks and attempt to answer this question, we inoculated juvenile mallards with 23 different H5N1 influenza viruses isolated in Asia between 2003 and 2004. All virus isolates replicated efficiently in inoculated ducks, and 22 were transmitted to susceptible contacts. Viruses replicated to higher levels in the trachea than in the cloaca of both inoculated and contact birds, suggesting that the digestive tract is not the main site of H5N1 influenza virus replication in ducks and that the fecal-oral route may no longer be the main transmission path. The virus isolates' pathogenicities varied from completely nonpathogenic to highly lethal and were positively correlated with tracheal virus titers. Nevertheless, the eight virus isolates that were nonpathogenic in ducks replicated and transmitted efficiently to naïve contacts, suggesting that highly pathogenic H5N1 viruses causing minimal signs of disease in ducks can propagate silently and efficiently among domestic and wild ducks in Asia and that they represent a serious threat to human and veterinary public health.
Figures

References
-
- Alexander, D. J., G. Parsons, and R. J. Manvell. 1986. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol. 15:647-662. - PubMed
-
- Capua, I., and F. Mutinelli. 2001. Mortality in Muscovy ducks (Cairina moschata) and domestic geese (Anser anser var. domestica) associated with natural infection with a highly pathogenic avian influenza virus of H7N1 subtype. Avian Pathol. 30:179-183. - PubMed
-
- Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. de Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

