Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle
- PMID: 16103225
- PMCID: PMC1283094
- DOI: 10.1083/jcb.200504131
Sgk3 links growth factor signaling to maintenance of progenitor cells in the hair follicle
Abstract
Tyrosine kinase growth factor receptor signaling influences proliferation, survival, and apoptosis. Hair follicles undergo cycles of proliferation and apoptotic regression, offering an excellent paradigm to study how this transition is governed. Several factors are known to affect the hair cycle, but it remains a mystery whether Akt kinases that are downstream of growth factor signaling impact this equilibrium. We now show that an Akt relative, Sgk (serum and glucocorticoid responsive kinase) 3, plays a critical role in this process. Hair follicles of mice lacking Sgk3 fail to mature normally. Proliferation is reduced, apoptosis is increased, and follicles prematurely regress. Maintenance of the pool of transiently amplifying matrix cells is impaired. Intriguingly, loss of Sgk3 resembles the gain of function of epidermal growth factor signaling. Using cultured primary keratinocytes, we find that Sgk3 functions by negatively regulating phosphatidylinositol 3 kinase signaling. Our results reveal a novel and important function for Sgk3 in controlling life and death in the hair follicle.
Figures
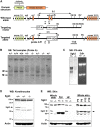

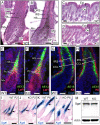

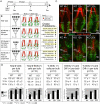

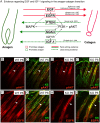

References
-
- Blanpain, C., W.E. Lowry, A. Geoghegan, L. Polak, and E. Fuchs. 2004. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 118:635–648. - PubMed
-
- Bol, D.K., K. Kiguchi, I. Gimenez-Conti, T. Rupp, and J. DiGiovanni. 1997. Overexpression of insulin-like growth factor-1 induces hyperplasia, dermal abnormalities, and spontaneous tumor formation in transgenic mice. Oncogene. 14:1725–1734. - PubMed
-
- DasGupta, R., and E. Fuchs. 1999. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 126:4557–4568. - PubMed
-
- Datta, S.R., A. Brunet, and M.E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

