An ECT2-centralspindlin complex regulates the localization and function of RhoA
- PMID: 16103226
- PMCID: PMC2171506
- DOI: 10.1083/jcb.200501097
An ECT2-centralspindlin complex regulates the localization and function of RhoA
Abstract
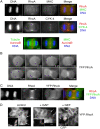
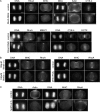
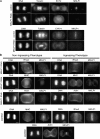
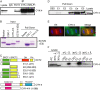
In anaphase, the spindle dictates the site of contractile ring assembly. Assembly and ingression of the contractile ring involves activation of myosin-II and actin polymerization, which are triggered by the GTPase RhoA. In many cells, the central spindle affects division plane positioning via unknown molecular mechanisms. Here, we dissect furrow formation in human cells and show that the RhoGEF ECT2 is required for cortical localization of RhoA and contractile ring assembly. ECT2 concentrates on the central spindle by binding to centralspindlin. Depletion of the centralspindlin component MKLP1 prevents central spindle localization of ECT2; however, RhoA, F-actin, and myosin still accumulate on the equatorial cell cortex. Depletion of the other centralspindlin component, CYK-4/MgcRacGAP, prevents cortical accumulation of RhoA, F-actin, and myosin. CYK-4 and ECT2 interact, and this interaction is cell cycle regulated via ECT2 phosphorylation. Thus, central spindle localization of ECT2 assists division plane positioning and the CYK-4 subunit of centralspindlin acts upstream of RhoA to promote furrow assembly.
Figures






References
-
- Aghazadeh, B., W.E. Lowry, X.Y. Huang, and M.K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 102:625–633. - PubMed
-
- Alberts, A.S. 2001. Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276:2824–2830. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

