An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway
- PMID: 16103228
- PMCID: PMC2171494
- DOI: 10.1083/jcb.200505128
An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway
Abstract
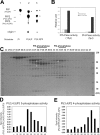
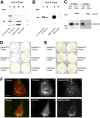
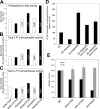
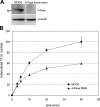
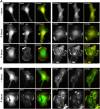
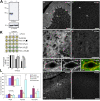
Generation and turnover of phosphoinositides (PIs) must be coordinated in a spatial- and temporal-restricted manner. The small GTPase Rab5 interacts with two PI 3-kinases, Vps34 and PI3Kbeta, suggesting that it regulates the production of 3-PIs at various stages of the early endocytic pathway. Here, we discovered that Rab5 also interacts directly with PI 5- and PI 4-phosphatases and stimulates their activity. Rab5 regulates the production of phosphatidylinositol 3-phosphate (PtdIns[3]P) through a dual mechanism, by directly phosphorylating phosphatidylinositol via Vps34 and by a hierarchical enzymatic cascade of phosphoinositide-3-kinasebeta (PI3Kbeta), PI 5-, and PI 4-phosphatases. The functional importance of such an enzymatic pathway is demonstrated by the inhibition of transferrin uptake upon silencing of PI 4-phosphatase and studies in weeble mutant mice, where deficiency of PI 4-phosphatase causes an increase of PtdIns(3,4)P2 and a reduction in PtdIns(3)P. Activation of PI 3-kinase at the plasma membrane is accompanied by the recruitment of Rab5, PI 4-, and PI 5-phosphatases to the cell cortex. Our data provide the first evidence for a dual role of a Rab GTPase in regulating both generation and turnover of PIs via PI kinases and phosphatases to coordinate signaling functions with organelle homeostasis.
Figures

References
-
- Attree, O., I.M. Olivos, I. Okabe, L.C. Bailey, D.L. Nelson, R.A. Lewis, R.R. McInnes, and R.L. Nussbaum. 1992. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 358:239–242. - PubMed
-
- Backman, S.A., V. Stambolic, A. Suzuki, J. Haight, A. Elia, J. Pretorius, M.S. Tsao, P. Shannon, B. Bolon, G.O. Ivy, and T.W. Mak. 2001. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte-Duclos disease. Nat. Genet. 29:396–403. - PubMed
-
- Choudhury, R., A. Diao, F. Zhang, E. Eisenberg, A. Saint-Pol, C. Williams, A. Konstantakopoulos, J. Lucocq, L. Johannes, C. Rabouille, et al. 2005. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol. Biol. Cell. 10.1091/mbc.E05-02-0120. - DOI - PMC - PubMed
-
- Christoforidis, S., H. McBride, D. Burgoyne, and M. Zerial. 1999. a. The Rab5 effector EEA1 is a core component of endosome docking. Nature. 397:621–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

