Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors
- PMID: 16103229
- PMCID: PMC2171515
- DOI: 10.1083/jcb.200503140
Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors
Abstract
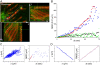
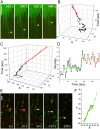
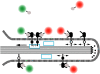
ErbB1 receptors situated on cellular filopodia undergo systematic retrograde transport after binding of the epidermal growth factor (EGF) and activation of the receptor tyrosine kinase. Specific inhibitors of the erbB1 receptor tyrosine kinase as well as cytochalasin D, a disruptor of the actin cytoskeleton, abolish transport but not free diffusion of the receptor-ligand complex. Diffusion constants and transport rates were determined with single molecule sensitivity by tracking receptors labeled with EGF conjugated to fluorescent quantum dots. Retrograde transport precedes receptor endocytosis, which occurs at the base of the filopodia. Initiation of transport requires the interaction and concerted activation of at least two liganded receptors and proceeds at a constant rate mediated by association with actin. These findings suggest a mechanism by which filopodia detect the presence and concentration of effector molecules far from the cell body and mediate cellular responses via directed transport of activated receptors.
Figures




References
-
- Brock, R., and T.M. Jovin. 2001. Heterogeneity of signal transduction at the subcellular level: microsphere-based focal EGF receptor activation and stimulation of Shc translocation. J. Cell Sci. 114:2437–2447. - PubMed
-
- Brock, R., and T.M. Jovin. 2003. Quantitative image analysis of cellular protein translocation induced by magnetic microspheres: application to the EGF receptor. Cytometry. 52:1–11. - PubMed
-
- Brock, R., I.H.L. Hamelers, and T.M. Jovin. 1999. a. Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry. 35:353–362. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

