Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal
- PMID: 16103361
- PMCID: PMC1189351
- DOI: 10.1073/pnas.0505714102
Mutant mice lacking acetyl-CoA carboxylase 1 are embryonically lethal
Abstract
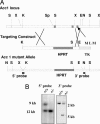
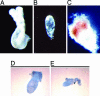
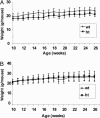
Acetyl-CoA carboxylases (ACC1 and ACC2) catalyze the carboxylation of acetyl-CoA to form malonyl-CoA, an intermediate metabolite that plays a pivotal role in the regulation of fatty acid metabolism. We previously reported that ACC2 null mice are viable, and that ACC2 plays an important role in the regulation of fatty acid oxidation through the inhibition of carnitine palmitoyltransferase I, a mitochondrial component of the fatty-acyl shuttle system. Herein, we used gene targeting to knock out the ACC1 gene. The heterozygous mutant mice (Acc1(+/-)) had normal fertility and lifespans and maintained a similar body weight to that of their wild-type cohorts. The mRNA level of ACC1 in the tissues of Acc1(+/-) mice was half that of the wild type; however, the protein level of ACC1 and the total malonyl-CoA level were similar. In addition, there was no difference in the acetate incorporation into fatty acids nor in the fatty acid oxidation between the hepatocytes of Acc1(+/-) mice and those of the wild type. In contrast to Acc2(-/-) mice, Acc1(-/-) mice were not detected after mating. Timed pregnancies of heterozygotes revealed that Acc(-/-) embryos are already undeveloped at embryonic day (E)7.5, they die by E8.5, and are completely resorbed at E11.5. Our previous results of the ACC2 knockout mice and current studies of ACC1 knockout mice further confirm our hypotheses that malonyl-CoA exists in two independent pools, and that ACC1 and ACC2 have distinct roles in fatty acid metabolism.
Figures


References
-
- Prentki, M., Joly, E., El-Assaad, W. & Roduit, R. (2002) Diabetes 51, S405–S413. - PubMed
-
- Roduit, R., Nolan, C., Alarcon, C., Moore, P., Barbeau, A., Delghingaro-Augusto, V., Przybykowski, E., Morin, J., Masse, F., Massie, B., et al. (2004) Diabetes 53, 1007–1019. - PubMed
-
- McGarry, J. D. & Brown, N. F. (1997) Eur. J. Biochem. 244, 1–14. - PubMed
-
- Abu-Elheiga, L., Matzuk, M. M., Abo-Hashema, K. A. H. & Wakil, S. J. (2001) Science 291, 2613–2616. - PubMed
-
- Lopaschuk, G. & Gamble, J. (1994) Can. J. Physiol. Pharmacol. 72, 1101–1109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

