An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation
- PMID: 16103363
- PMCID: PMC1189320
- DOI: 10.1073/pnas.0503723102
An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation
Abstract
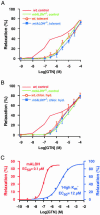
The identity of the cellular mechanisms through which nitroglycerin (glyceryl trinitrate, GTN) elicits nitric oxide (NO)-based signaling to dilate blood vessels remains one of the longest standing foci of investigation and sources of controversy in cardiovascular biology. Recent evidence suggests an unexpected role for mitochondria. We show here that bioconversion by mitochondria of clinically relevant concentrations of GTN results in activation of guanylate cyclase, production of cGMP, vasodilation in vitro, and lowered blood pressure in vivo, which are eliminated by genetic deletion of the mitochondrial aldehyde dehydrogenase (mtALDH). In contrast, generation of vasoactivity from alternative nitro(so)-vasodilators is unaffected. In mtALDH(-/-) mice and their isolated vascular tissue, GTN bioactivity can still be generated, but only at substantially higher concentrations of GTN and by a mechanism that does not exhibit tolerance. Thus, mtALDH is necessary and sufficient for vasoactivity derived from therapeutic levels of GTN, and, more generally, mitochondria can serve as a source of NO-based cellular signals that may originate independently of NO synthase activity.
Figures

References
-
- Gruetter, C. A., Gruetter, D. Y., Lyon, J. E., Kadowitz, P. J. & Ignarro L. J. (1981) J. Pharmacol. Exp. Ther. 219, 181-186. - PubMed
-
- Bennet, B. M., McDonald, B. J., Nigam, R. & Simon, W. C. (1994) Trends Pharmacol. Sci. 15, 245-249. - PubMed
-
- Fung, H.-L. (2004) Ann. Rev. Pharmacol. Toxicol. 44, 67-85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

