A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid
- PMID: 16103367
- PMCID: PMC1189297
- DOI: 10.1073/pnas.0406731102
A role for transferrin receptor in triggering apoptosis when targeted with gambogic acid
Abstract
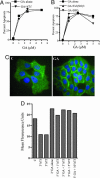
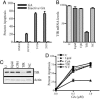
Transferrin receptor (TfR) has been shown to be significantly overexpressed in different types of cancers. We discovered TfR as a target for gambogic acid (GA), used in traditional Chinese medicine and a previously undiscovered link between TfR and the rapid activation of apoptosis. The binding site of GA on TfR is independent of the transferrin binding site, and it appears that GA potentially inhibits TfR internalization. Down-regulation of TfR by RNA interference decreases sensitivity to GA-induced apoptosis, further supporting TfR as the primary GA receptor. In summary, GA binding to TfR induces a unique signal leading to rapid apoptosis of tumor cells. These results suggest that GA may provide an additional approach for targeting the TfR and its use in cancer therapy.
Figures




Similar articles
-
Mechanisms of gambogic acid-induced apoptosis in non-small cell lung cancer cells in relation to transferrin receptors.J Chemother. 2009 Dec;21(6):666-72. doi: 10.1179/joc.2009.21.6.666. J Chemother. 2009. PMID: 20071291
-
Gambogic acid, a novel ligand for transferrin receptor, potentiates TNF-induced apoptosis through modulation of the nuclear factor-kappaB signaling pathway.Blood. 2007 Nov 15;110(10):3517-25. doi: 10.1182/blood-2007-03-079616. Epub 2007 Aug 2. Blood. 2007. PMID: 17673602 Free PMC article.
-
Gambogic acid induces apoptosis by regulating the expression of Bax and Bcl-2 and enhancing caspase-3 activity in human malignant melanoma A375 cells.Int J Dermatol. 2009 Feb;48(2):186-92. doi: 10.1111/j.1365-4632.2009.03946.x. Int J Dermatol. 2009. PMID: 19200201
-
The transferrin receptor and the targeted delivery of therapeutic agents against cancer.Biochim Biophys Acta. 2012 Mar;1820(3):291-317. doi: 10.1016/j.bbagen.2011.07.016. Epub 2011 Aug 5. Biochim Biophys Acta. 2012. PMID: 21851850 Free PMC article. Review.
-
The transferrin receptor part I: Biology and targeting with cytotoxic antibodies for the treatment of cancer.Clin Immunol. 2006 Nov;121(2):144-58. doi: 10.1016/j.clim.2006.06.010. Epub 2006 Aug 10. Clin Immunol. 2006. PMID: 16904380 Review.
Cited by
-
Paclitaxel-Fe3O4 nanoparticles inhibit growth of CD138(-) CD34(-) tumor stem-like cells in multiple myeloma-bearing mice.Int J Nanomedicine. 2013;8:1439-49. doi: 10.2147/IJN.S38447. Epub 2013 Apr 12. Int J Nanomedicine. 2013. PMID: 23610522 Free PMC article.
-
Gambogic acid triggers vacuolization-associated cell death in cancer cells via disruption of thiol proteostasis.Cell Death Dis. 2019 Feb 22;10(3):187. doi: 10.1038/s41419-019-1360-4. Cell Death Dis. 2019. PMID: 30796201 Free PMC article.
-
Single-walled carbon nanotube and graphene nanodelivery of gambogic acid increases its cytotoxicity in breast and pancreatic cancer cells.J Appl Toxicol. 2014 Nov;34(11):1188-99. doi: 10.1002/jat.3018. Epub 2014 Sep 12. J Appl Toxicol. 2014. PMID: 25220893 Free PMC article.
-
Gambogic acid inhibits STAT3 phosphorylation through activation of protein tyrosine phosphatase SHP-1: potential role in proliferation and apoptosis.Cancer Prev Res (Phila). 2011 Jul;4(7):1084-94. doi: 10.1158/1940-6207.CAPR-10-0340. Epub 2011 Apr 13. Cancer Prev Res (Phila). 2011. Retraction in: Cancer Prev Res (Phila). 2018 Sep;11(9):593. doi: 10.1158/1940-6207.CAPR-18-0235. PMID: 21490133 Free PMC article. Retracted.
-
TfR1 interacts with the IKK complex and is involved in IKK-NF-κB signalling.Biochem J. 2013 Jan 1;449(1):275-84. doi: 10.1042/BJ20120625. Biochem J. 2013. PMID: 23016877 Free PMC article.
References
-
- Bennett, M. J, Lebron, J. A., Bjorkman, P. J. (2000) Nature 403, 46–53. - PubMed
-
- Lawrence, C., Ray, S., Babyonyshev, M., Galluser, R., Borhani, D. & Harrison, S. (1999) Science 286, 779–782. - PubMed
-
- Ryschich, E., Huszty, G., Knaebel, H., Hartel, M., Buchler, M. & Schmidt, J. (2004) Eur. J. Cancer 40, 1418–1422. - PubMed
-
- Szekeres, T., Sedlak, J. & Novotny, L. (2002) Curr. Med. Chem. 9, 759–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

