Obstructive nephropathy: insights from genetically engineered animals
- PMID: 16105023
- PMCID: PMC1885837
- DOI: 10.1111/j.1523-1755.2005.00486.x
Obstructive nephropathy: insights from genetically engineered animals
Abstract
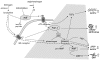
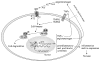
Congenital obstructive nephropathy is the primary cause for end-stage renal disease (ESRD) in children. An increasingly used animal model of obstructive nephropathy is unilateral ureteral obstruction (UUO). This model mimics, in an accelerated manner, the different stages of obstructive nephropathy leading to tubulointerstitial fibrosis: cellular infiltration, tubular proliferation and apoptosis, epithelial-mesenchymal transition (EMT), (myo)fibroblast accumulation, increased extracellular matrix (ECM) deposition, and tubular atrophy. During the last decade genetically modified animals are increasingly used to study the development of obstructive nephropathy. Although the use of these animals (mainly knockouts) has highlighted some pitfalls of this approach (compensation by closely related gene products, absence of temporal knockouts) it has brought important information about the role of specific gene-products in the pathogenesis of obstructive nephropathy. Besides confirming the important pathologic role for angiotensin II (Ang II) and transforming growth factor-beta (TGF-beta) in obstructive nephropathy, these animals have shown the complexity of the development of tubulointerstitial fibrosis involving a large number of closely functionally related molecules. More interestingly, the use of these animals has led to the discovery of unexpected and contradictory roles (both potentially pro- and antifibrotic) for Ang II, for ECM degrading enzymes matrix metalloproteinase 9 (MMP-9) and tissue plasminogen activators (PAs), for plasminogen activator inhibitor 1 (PAI-1), and for the adhesion molecule osteopontin (OPN) in obstructive nephropathy. Further use of these animals, especially in combination with pharmacologic tools, should help to better identify potential antifibrotic strategies in obstructive nephropathy.
Figures




References
-
- Roth KS, Koo HP, Spottswood SE, Chan JC. Obstructive uropathy: an important cause of chronic renal failure in children. Clin Pediatr. 2002;45:309–314. - PubMed
-
- Benfield MR, McDonald RA, Bartosh S, et al. Changing trends in pediatric transplantation: 2001 Annual Report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant. 2003;7:321–335. - PubMed
-
- U.S. Renal Data System. USRDS 2003 Annual Data Report: Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2003.
-
- Klahr S. Obstructive nephropathy. Kidney Int. 1998;54:286–300. - PubMed
-
- Chevalier RL. Molecular and cellular pathophysiology of obstructive nephropathy. Pediatr Nephrol. 1999;13:612–619. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

