Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization
- PMID: 16105948
- PMCID: PMC1194917
- DOI: 10.1073/pnas.0503270102
Enhancing the anticancer properties of cardiac glycosides by neoglycorandomization
Abstract
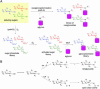
Glycosylated natural products are reliable platforms for the development of many front-line drugs, yet our understanding of the relationship between attached sugars and biological activity is limited by the availability of convenient glycosylation methods. When a universal chemical glycosylation method that employs reducing sugars and requires no protection or activation is used, the glycorandomization of digitoxin leads to analogs that display significantly enhanced potency and tumor specificity and suggests a divergent mechanistic relationship between cardiac glycoside-induced cytotoxicity and Na+/K+-ATPase inhibition. This report highlights the remarkable advantages of glycorandomization as a powerful tool in glycobiology and drug discovery.
Figures
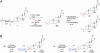
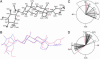

References
-
- Sears, P. & Wong, C.-H. (2001) Science 291, 2344–2350. - PubMed
-
- Bertozzi, C. R. & Kiessling, L. L. (2001) Science 291, 2357–2364. - PubMed
-
- Zhang, Z., Gildersleeve, J., Yang, Y.-Y., Xu, R., Loo, J. A., Uryu, S., Wong, C.-H. & Schultz, P. G. (2004) Science 303, 371–373. - PubMed
-
- Prescher, J. A., Dube, D. H. & Bertozzi, C. R. (2004) Nature 430, 873–877. - PubMed
-
- Clardy, J. & Walsh, C. (2004) Nature 432, 829–837. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

