p130Cas mediates the transforming properties of the anaplastic lymphoma kinase
- PMID: 16105984
- PMCID: PMC1895100
- DOI: 10.1182/blood-2005-03-1204
p130Cas mediates the transforming properties of the anaplastic lymphoma kinase
Erratum in
- Blood. 2009 Sep 24;114(13):2851
Abstract
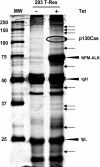
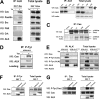
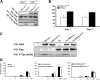
Translocations of the anaplastic lymphoma kinase (ALK) gene have been described in anaplastic large-cell lymphomas (ALCLs) and in stromal tumors. The most frequent translocation, t(2;5), generates the fusion protein nucleophosmin (NPM)-ALK with intrinsic tyrosine kinase activity. Along with transformation, NPM-ALK induces morphologic changes in fibroblasts and lymphoid cells, suggesting a direct role of ALK in cell shaping. In this study, we used a mass-spectrometry-based proteomic approach to search for proteins involved in cytoskeleton remodeling and identified p130Cas (p130 Crk-associated substrate) as a novel interactor of NPM-ALK. In 293 cells and in fibroblasts as well as in human ALK-positive lymphoma cell lines, NPM-ALK was able to bind p130Cas and to induce its phosphorylation. Both of the effects were dependent on ALK kinase activity and on the adaptor protein growth factor receptor-bound protein 2 (Grb2), since no binding or phosphorylation was found with the kinase-dead mutant NPM-ALK(K210R) or in the presence of a Grb2 dominant-negative protein. Phosphorylation of p130Cas by NPM-ALK was partially independent from Src (tyrosine kinase pp60c-src) kinase activity, as it was still detectable in Syf-/- cells. Finally, p130Cas-/- (also known as Bcar1-/-) fibroblasts expressing NPM-ALK showed impaired actin filament depolymerization and were no longer transformed compared with wild-type cells, indicating an essential role of p130Cas activation in ALK-mediated transformation.
Figures

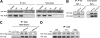


References
-
- Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science. 1994;263: 1281-1284. - PubMed
-
- Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56: 379-390. - PubMed
-
- Okuda M. The role of nucleophosmin in centrosome duplication. Oncogene. 2002;21: 6170-6174. - PubMed
-
- Kurki S, Peltonen K, Latonen L, et al. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell. 2004;5: 465-475. - PubMed
-
- Chan PK, Chan FY. Nucleophosmin/B23 (NPM) oligomer is a major and stable entity in HeLa cells. Biochim Biophys Acta. 1995;1262: 37-42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

