Mortality following development of breast cancer while using oestrogen or oestrogen plus progestin: a computer record-linkage study
- PMID: 16106246
- PMCID: PMC2361586
- DOI: 10.1038/sj.bjc.6602701
Mortality following development of breast cancer while using oestrogen or oestrogen plus progestin: a computer record-linkage study
Abstract
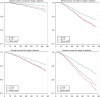
The literature on the relationship between breast cancer mortality and postmenopausal oestrogen and combined oestrogen/progestin therapy is seemingly contradictory. This study explored survival after exposure to oestrogen or oestrogen plus progestin at or in the year prior to breast cancer diagnosis. Information on patients first diagnosed with invasive breast cancer between 1993 and 1998 was linked with outpatient pharmacy data from 1992 to 2000. Patients were classified according to use of oestrogen alone or oestrogen plus progestin at or in the year prior to diagnosis. Compared to nonusers, and adjusting for age at diagnosis, race/ethnicity, tumour size and grade, oestrogen receptor status, surgery status, and chemotherapy and hormone therapy for breast cancer treatment, oestrogen plus progestin users had lower all-cause mortality (stage I hazard ratio (HR) = 0.69, 95% confidence interval (CI)= 0.48-0.99; stage II HR = 0.53, 95% CI = 0.39-0.72) and breast cancer mortality (stage I HR = 0.52, 95% CI = 0.26-1.04; stage II HR = 0.69, 95% CI = 0.48-0.98). Oestrogen users experienced little or no survival benefit for all-cause mortality (stage I HR = 1.04, 95% CI = 0.77-1.42; stage II HR = 0.86, 95% CI = 0.65-1.14) or breast cancer mortality (stage I HR = 1.23, 95% CI 0.72-2.10; stage II HR = 1.01, 95% CI 0.72-1.41). Our findings suggest, relative to nonusers, a lower risk of death from all causes and from breast cancer in patients who were diagnosed with breast cancer while exposed to oestrogen plus progestin, but not in patients exposed to oestrogen only.
Figures
Similar articles
-
Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study.J Natl Cancer Inst. 2013 Apr 17;105(8):526-35. doi: 10.1093/jnci/djt043. Epub 2013 Mar 29. J Natl Cancer Inst. 2013. PMID: 23543779 Free PMC article.
-
Postmenopausal estrogen-containing hormone therapy and the risk of breast cancer.Obstet Gynecol. 2009 Jan;113(1):74-80. doi: 10.1097/AOG.0b013e31818fdde4. Obstet Gynecol. 2009. PMID: 19104362
-
Mammography use, breast cancer stage at diagnosis, and survival among older women.J Am Geriatr Soc. 2000 Oct;48(10):1226-33. doi: 10.1111/j.1532-5415.2000.tb02595.x. J Am Geriatr Soc. 2000. PMID: 11037009
-
Breast cancer risk with postmenopausal hormonal treatment.Hum Reprod Update. 2005 Nov-Dec;11(6):545-60. doi: 10.1093/humupd/dmi028. Epub 2005 Sep 8. Hum Reprod Update. 2005. PMID: 16150813 Review.
-
Exogenous progestins and breast cancer.Epidemiol Rev. 1993;15(1):98-107. doi: 10.1093/oxfordjournals.epirev.a036120. Epidemiol Rev. 1993. PMID: 8405216 Review.
Cited by
-
Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women's Health Initiative randomised placebo-controlled trial.Lancet Oncol. 2012 May;13(5):476-86. doi: 10.1016/S1470-2045(12)70075-X. Epub 2012 Mar 7. Lancet Oncol. 2012. PMID: 22401913 Free PMC article. Clinical Trial.
-
Menopausal hormone therapy and incidence, mortality, and survival of breast cancer subtypes: a prospective cohort study.Breast Cancer Res. 2024 Nov 4;26(1):151. doi: 10.1186/s13058-024-01897-4. Breast Cancer Res. 2024. PMID: 39497219 Free PMC article.
-
Menopausal hormone therapy and breast cancer mortality: clinical implications.Ther Adv Drug Saf. 2015 Apr;6(2):45-56. doi: 10.1177/2042098614568300. Ther Adv Drug Saf. 2015. PMID: 25922653 Free PMC article. Review.
-
Estrogen plus progestin and breast cancer incidence and mortality in the Women's Health Initiative Observational Study.J Natl Cancer Inst. 2013 Apr 17;105(8):526-35. doi: 10.1093/jnci/djt043. Epub 2013 Mar 29. J Natl Cancer Inst. 2013. PMID: 23543779 Free PMC article.
-
Prognostic Impact of Menopausal Hormone Therapy in Breast Cancer Differs According to Tumor Characteristics and Treatment.Front Oncol. 2020 Feb 6;10:80. doi: 10.3389/fonc.2020.00080. eCollection 2020. Front Oncol. 2020. PMID: 32117735 Free PMC article.
References
-
- Antonie C, Liebens F, Carly B, Pastijn A, Rozenbarg S (2004) Influence of HRT on prognostic factors for breast cancer: a systematic review after the Women's Health Initiative trial. Hum Reprod 19: 741–756 - PubMed
-
- Barrett-Connor E (1991) Postmenopausal estrogen and prevention bias. Ann Intern Med 115: 455–456 - PubMed
-
- Beral V (2003) Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362: 419–427 - PubMed
-
- Bergkvist L, Adami HO, Persson I, Bergstrom R, Krusemo UB (1989) Prognosis after breast cancer diagnosis in women exposed to estrogen and estrogen–progestogen replacement therapy. Am J Epidemiol 130: 221–228 - PubMed
-
- Bundred NJ, Morris J (2003) Breast cancer and hormone-replacement therapy: the Million Women Study. Lancet 362: 1329. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

