The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation
- PMID: 16107639
- PMCID: PMC6725402
- DOI: 10.1523/JNEUROSCI.2010-05.2005
The two isoforms of the Caenorhabditis elegans leukocyte-common antigen related receptor tyrosine phosphatase PTP-3 function independently in axon guidance and synapse formation
Abstract
Leukocyte-common antigen related (LAR)-like phosphatase receptors are conserved cell adhesion molecules that function in multiple developmental processes. The Caenorhabditis elegans ptp-3 gene encodes two LAR family isoforms that differ in the extracellular domain. We show here that the long isoform, PTP-3A, localizes specifically at synapses and that the short isoform, PTP-3B, is extrasynaptic. Mutations in ptp-3 cause defects in axon guidance that can be rescued by PTP-3B but not by PTP-3A. Mutations that specifically affect ptp-3A do not affect axon guidance but instead cause alterations in synapse morphology. Genetic double-mutant analysis is consistent with ptp-3A acting with the extracellular matrix component nidogen, nid-1, and the intracellular adaptor alpha-liprin, syd-2. nid-1 and syd-2 are required for the recruitment and stability of PTP-3A at synapses, and mutations in ptp-3 or nid-1 result in aberrant localization of SYD-2. Overexpression of PTP-3A is able to bypass the requirement for nid-1 for the localization of SYD-2 and RIM. We propose that PTP-3A acts as a molecular link between the extracellular matrix and alpha-liprin during synaptogenesis.
Figures

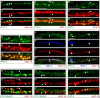

 ), or defasciculation (▪). Information on specific axon outgrowth phenotypes is in Table 2. L/R, Left/right.
), or defasciculation (▪). Information on specific axon outgrowth phenotypes is in Table 2. L/R, Left/right.
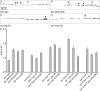
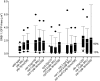


References
-
- Altun-Gultekin Z, Andachi Y, Tsalik EL, Pilgrim D, Kohara Y, Hobert O (2001) A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans Development 128: 1951-1969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
