Mechanism of von Hippel-Lindau protein-mediated suppression of nuclear factor kappa B activity
- PMID: 16107702
- PMCID: PMC1190288
- DOI: 10.1128/MCB.25.17.7546-7556.2005
Mechanism of von Hippel-Lindau protein-mediated suppression of nuclear factor kappa B activity
Abstract
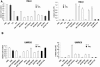
Biallelic inactivating mutations of the von Hippel-Lindau tumor suppressor gene (VHL) are a hallmark of clear cell renal cell carcinoma (CCRCC), the most common histologic subtype of RCC. Biallelic VHL loss results in accumulation of hypoxia-inducible factor alpha (HIFalpha). Restoring expression of the wild-type protein encoded by VHL (pVHL) in tumors with biallelic VHL inactivation (VHL(-)(/)(-)) suppresses tumorigenesis, and pVHL-mediated degradation of HIFalpha is necessary and sufficient for VHL-mediated tumor suppression. The downstream targets of HIFalpha that promote renal carcinogenesis have not been completely elucidated. Recently, VHL loss was shown to activate nuclear factor kappa B (NF-kappaB), a family of transcription factors that promotes tumor growth. Here we show that VHL loss drives NF-kappaB activation by resulting in HIFalpha accumulation, which induces expression of transforming growth factor alpha, with consequent activation of an epidermal growth factor receptor/phosphatidylinositol-3-OH kinase/protein kinase B (AKT)/IkappaB-kinase alpha/NF-kappaB signaling cascade. We also show that components of this signaling pathway promote the growth of VHL(-)(/)(-) tumor cells. Members of this pathway represent viable drug targets in VHL(-)(/)(-) tumors, such as those associated with CCRCC.
Figures



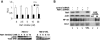

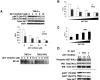
References
-
- An, J., M. Fisher, and M. B. Rettig. 2005. VHL expression in renal cell carcinoma sensitizes to bortezomib (PS-341) through an NF-kappaB-dependent mechanism. Oncogene 24:1563-1570. - PubMed
-
- An, J., Y. Sun, M. Fisher, and M. B. Rettig. 2004. Maximal apoptosis of renal cell carcinoma by the proteasome inhibitor bortezomib is nuclear factor-kappaB dependent. Mol. Cancer Ther. 3:727-736. - PubMed
-
- Atlas, I., J. Mendelsohn, J. Baselga, W. R. Fair, H. Masui, and R. Kumar. 1992. Growth regulation of human renal carcinoma cells: role of transforming growth factor alpha. Cancer Res. 52:3335-3339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
