Structural characterization of the histone variant macroH2A
- PMID: 16107708
- PMCID: PMC1190287
- DOI: 10.1128/MCB.25.17.7616-7624.2005
Structural characterization of the histone variant macroH2A
Abstract
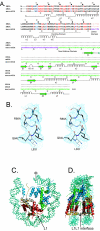
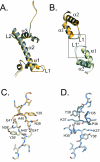
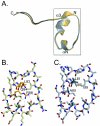
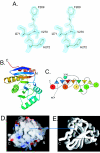
macroH2A is an H2A variant with a highly unusual structural organization. It has a C-terminal domain connected to the N-terminal histone domain by a linker. Crystallographic and biochemical studies show that changes in the L1 loop in the histone fold region of macroH2A impact the structure and potentially the function of nucleosomes. The 1.6-A X-ray structure of the nonhistone region reveals an alpha/beta fold which has previously been found in a functionally diverse group of proteins. This region associates with histone deacetylases and affects the acetylation status of nucleosomes containing macroH2A. Thus, the unusual domain structure of macroH2A integrates independent functions that are instrumental in establishing a structurally and functionally unique chromatin domain.
Figures


References
-
- Abbott, D. W., V. S. Ivanova, X. Wang, W. M. Bonner, and J. Ausio. 2001. Characterization of the stability and folding of H2A.Z chromatin particles: implications for transcriptional activation. J. Biol. Chem. 276:41945-41949. - PubMed
-
- Ahmad, K., and S. Henikoff. 2002. The histone variant H3.3 marks active chromatin by replication- independent nucleosome assembly. Mol. Cell 9:1191-1200. - PubMed
-
- Allen, M. D., A. M. Buckle, S. C. Cordell, J. Lowe, and M. Bycroft. 2003. The crystal structure of AF1521 a protein from Archaeoglobus fulgidus with homology to the non-histone domain of macroH2A. J. Mol. Biol. 330:503-511. - PubMed
-
- Angelov, D., A. Molla, P. Y. Perche, F. Hans, J. Cote, S. Khochbin, P. Bouvet, and S. Dimitrov. 2003. The histone variant MacroH2A interferes with transcription factor binding and SWI/SNF nucleosome remodeling. Mol. Cell 11:1033-1041. - PubMed
-
- Belotserkovskaya, R., S. Oh, V. A. Bondarenko, G. Orphanides, V. M. Studitsky, and D. Reinberg. 2003. FACT facilitates transcription-dependent nucleosome alteration. Science 301:1090-1093. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
