Breast cancer resistance protein: molecular target for anticancer drug resistance and pharmacokinetics/pharmacodynamics
- PMID: 16108826
- PMCID: PMC11158713
- DOI: 10.1111/j.1349-7006.2005.00081.x
Breast cancer resistance protein: molecular target for anticancer drug resistance and pharmacokinetics/pharmacodynamics
Abstract
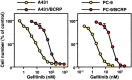
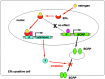
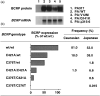
Breast cancer resistance protein (BCRP) is a half-molecule ATP-binding cassette transporter that forms a functional homodimer and pumps out various anticancer agents, such as 7-ethyl-10-hydroxycamptothecin, topotecan, mitoxantrone and flavopiridol, from cells. Estrogens, such as estrone and 17beta-estradiol, have been found to restore drug sensitivity levels in BCRP-transduced cells by increasing the cellular accumulation of such agents. Furthermore, synthetic estrogens, tamoxifen derivatives and phytoestrogens/flavonoids have now been identified that can effectively circumvent BCRP-mediated drug resistance. Transcellular transport experiments have shown that BCRP transports sulfated estrogens and various sulfated steroidal compounds, but not free estrogens. The kinase inhibitor gefitinib inhibited the transporter function of BCRP and reversed BCRP-mediated drug resistance both in vitro and in vivo. BCRP-transduced human epidermoid carcinoma A431 (A431/BCRP) and BCRP-transduced human non-small cell lung cancer PC-9 (PC-9/BCRP) cells showed gefitinib resistance. Physiological concentrations of estrogens (10-100 pM) reduced BCRP protein expression without affecting its mRNA levels. Two functional polymorphisms of the BCRP gene have been identified. The C376T (Q126Stop) polymorphism has a dramatic phenotype as active BCRP protein cannot be expressed from a C376T allele. The C421A (Q141K) polymorphism is also significant as Q141K-BCRP-transfected cells show markedly low protein expression levels and low-level drug resistance. Hence, individuals with C376T or C421A polymorphisms may express low levels of BCRP or none at all, resulting in hypersensitivity of normal cells to BCRP-substrate anticancer agents. In summary, both modulators of BCRP and functional single nucleotide polymorphisms within the BCRP gene affect the transporter function of the protein and thus can modulate drug sensitivity and substrate pharmacokinetics and pharmacodynamics in affected cells and individuals.
Figures
References
-
- Gottesman MM, Hrycyna CA, Schoenlein PV, Germann UA, Pastan I. Genetic analysis of the multidrug transporter. Annu Rev Genet 1995; 29: 607–49. - PubMed
-
- Chen CJ, Chin JE, Ueda K et al. Internal duplication and homology with bacterial transport proteins in the mdr1 (P‐glycoprotein) gene from multidrug‐resistant human cells. Cell 1986; 47: 381–9. - PubMed
-
- Cole SP, Bhardwaj G, Gerlach JH et al. Overexpression of a transporter gene in a multidrug‐resistant human lung cancer cell line. Science 1992; 258: 1650–4. - PubMed
-
- Allikmets R, Schriml L, Hutchinson A, Romano‐Spica V, Dean M. A human placenta‐specific ATP‐binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 1998; 58: 5337–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

