A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins
- PMID: 16109166
- PMCID: PMC1208859
- DOI: 10.1186/1472-6750-5-22
A simplified immunoprecipitation method for quantitatively measuring antibody responses in clinical sera samples by using mammalian-produced Renilla luciferase-antigen fusion proteins
Abstract
Background: Assays detecting human antigen-specific antibodies are medically useful. However, the usefulness of existing simple immunoassay formats is limited by technical considerations such as sera antibodies to contaminants in insufficiently pure antigen, a problem likely exacerbated when antigen panels are screened to obtain clinically useful data.
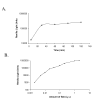
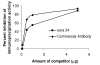
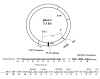
Results: We developed a novel and simple immunoprecipitation technology for identifying clinical sera containing antigen-specific antibodies and for generating quantitative antibody response profiles. This method is based on fusing protein antigens to an enzyme reporter, Renilla luciferase (Ruc), and expressing these fusions in mammalian cells, where mammalian-specific post-translational modifications can be added. After mixing crude extracts, sera and protein A/G beads together and incubating, during which the Ruc-antigen fusion become immobilized on the A/G beads, antigen-specific antibody is quantitated by washing the beads and adding coelenterazine substrate and measuring light production. We have characterized this technology with sera from patients having three different types of cancers. We show that 20-85% of these sera contain significant titers of antibodies against at least one of five frequently mutated and/or overexpressed tumor-associated proteins. Five of six colon cancer sera tested gave responses that were statistically significantly greater than the average plus three standard deviations of 10 control sera. The results of competition experiments, preincubating positive sera with unmodified E. coli-produced antigens, varied dramatically.
Conclusion: This technology has several advantages over current quantitative immunoassays including its relative simplicity, its avoidance of problems associated with E. coli-produced antigens and its use of antigens that can carry mammalian or disease-specific post-translational modifications. This assay should be generally useful for analyzing sera for antibodies recognizing any protein or its post-translational modifications.
Figures

References
-
- Wadhwa M, Skog AL, Bird C, Ragnhammar P, Lilljefors M, Gaines-Das R, Mellstedt H, Thorpe R. Immunogenicity of granulocyte-macrophage colony-stimulating factor (GM-CSF) products in patients undergoing combination therapy with GM-CSF. Clin Cancer Res. 1999;5:1353–1361. - PubMed
-
- Burbelo PD, Kisailus AE, Peck JW. Detecting protein-protein interactions using Renilla luciferase fusion proteins. Biotechniques. 2002;33:1044–1048. 1050. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

