CXCR4 and CCR5 shRNA transgenic CD34+ cell derived macrophages are functionally normal and resist HIV-1 infection
- PMID: 16109172
- PMCID: PMC1199620
- DOI: 10.1186/1742-4690-2-53
CXCR4 and CCR5 shRNA transgenic CD34+ cell derived macrophages are functionally normal and resist HIV-1 infection
Abstract
Background: Stable simultaneous knock down of the HIV-1 coreceptors CCR5 and CXCR4 is a promising strategy to protect cells from both R5 macrophage tropic and X4 T cell tropic as well as dual tropic viral infections. The potency of shRNAs in targeted gene silencing qualifies them as powerful tools for long term HIV gene therapy. Our previous work with a bispecific lentiviral vector containing CXCR4 and CCR5 shRNAs showed efficacy in down regulating both coreceptors and conferring viral resistance to both X4 and R5-tropic strains of HIV-1 in cultured cell lines. To extend these results to a stem cell gene therapy setting, here we show transduction of primary CD34+ hematopoietic progenitor cells to derive normal end stage cells that are resistant to HIV-1 infection.
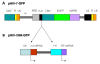
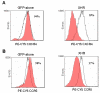
Results: The bispecific XHR lentiviral vector harboring CXCR4 and CCR5 shRNA expression cassettes was efficient in transducing CD34+ cells. The transduced cells gave rise to morphologically normal transgenic macrophages when cultured in cytokine media. There was a marked down regulation of both coreceptors in the stably transduced macrophages which showed resistance to both R5 and X4 HIV-1 strains upon in vitro challenge. Since off target effects by some shRNAs may have adverse effects on transgenic cells, the stably transduced macrophages were further analyzed to determine if they are phenotypically and functionally normal. FACS evaluation showed normal levels of the characteristic surface markers CD14, CD4, MHC class II, and B7.1. Phagocytic functions were also normal. The transgenic macrophages demonstrated normal abilities in up-regulating the costimulatory molecule B7.1 upon LPS stimulation. Furthermore, IL-1 and TNFalpha cytokine secretion in response to LPS stimulation was also normal. Thus, the transgenic macrophages appear to be phenotypically and functionally normal.
Conclusion: These studies have demonstrated for the first time that a bispecific lentiviral vector could be used to stably deliver shRNAs targeted to both CCR5 and CXCR4 coreceptors into CD34+ hematopoietic progenitor cells and derive transgenic macrophages. Transgenic macrophages with down regulated coreceptors were resistant to both R5 and X4 tropic HIV-1 infections. The differentiated cells were also phenotypically and functionally normal indicating no adverse effects of shRNAs on lineage specific differentiation of stem cells. It is now possible to construct gene therapeutic lentiviral vectors incorporating multiple shRNAs targeted to cellular molecules that aid in HIV-1 infection. Use of these vectors in a stem cell setting shows great promise for sustained HIV/AIDS gene therapy.
Figures
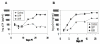

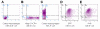

References
-
- Bonyhadi ML, Moss K, Voytovich A, Auten J, Kalfoglou C, Plavec I, Forestell S, Su L, Bohnlein E, Kaneshima H. RevM10-expressing T cells derived in vivo from transduced human hematopoietic stem-progenitor cells inhibit human immunodeficiency virus replication. J Virol. 1997;71:4707–4716. - PMC - PubMed
-
- Ding SF, Lombardi R, Nazari R, Joshi S. A combination anti-HIV-1 gene therapy approach using a single transcription unit that expresses antisense, decoy, and sense RNAs, and transdominant negative mutant Gag and Env proteins. Front Biosci. 2002;7:a15–28. - PubMed
-
- Akkina R, Banerjea A, bai J, Anderson J, Li MJ, Rossi J. siRNAs, ribozymes, and RNA decoys in modeling stem cell-based gene therapy for HIV/AIDS. Anticancer Res. 2003;23:1997–2006. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

