Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats
- PMID: 16109728
- PMCID: PMC1464188
- DOI: 10.1113/jphysiol.2005.093948
Mechanisms of target-cell specific short-term plasticity at Schaffer collateral synapses onto interneurones versus pyramidal cells in juvenile rats
Abstract
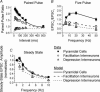
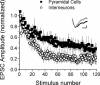
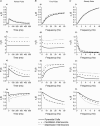
Although it is presynaptic, short-term plasticity has been shown at some synapses to depend upon the postsynaptic cell type. Previous studies have reported conflicting results as to whether Schaffer collateral axons have target-cell specific short-term plasticity. Here we investigate in detail the short-term dynamics of Schaffer collateral excitatory synapses onto CA1 stratum radiatum interneurones versus pyramidal cells in acute hippocampal slices from juvenile rats. In response to three stimulus protocols that invoke different forms of short-term plasticity, we find differences in some but not all forms of presynaptic short-term plasticity, and heterogeneity in the short term plasticity of synapses onto interneurones. Excitatory synapses onto the majority of interneurones had less paired-pulse facilitation than synapses onto pyramidal cells across a range of interpulse intervals (20-200 ms). Unlike synapses onto pyramidal cells, synapses onto most interneurones had very little facilitation in response to short high-frequency trains of five pulses at 5, 10 and 20 Hz, and depressed during trains at 50 Hz. However, the amount of high-frequency depression was not different between synapses onto pyramidal cells versus the majority of interneurones at steady state during 2-10 Hz trains. In addition, a small subset of interneurones (approximately 15%) had paired-pulse depression rather than paired-pulse facilitation, showed only depression in response to the high-frequency five pulse trains, and had more steady-state high-frequency depression than synapses onto pyramidal cells or the majority of interneurones. To investigate possible mechanisms for these differences in short-term plasticity, we developed a mechanistic mathematical model of neurotransmitter release that explicitly explores the contributions to different forms of short-term plasticity of the readily releasable vesicle pool size, release probability per vesicle, calcium-dependent facilitation, synapse inactivation following release, and calcium-dependent recovery from inactivation. Our model fits the responses of each of the three cell groups to the three different stimulus protocols with only two parameters that differ with cell group. The model predicts that the differences in short-term plasticity between synapses onto CA1 pyramidal cells and stratum radiatum interneurones are due to a higher initial release probability per vesicle and larger readily releasable vesicle pool size at synapses onto interneurones, resulting in a higher initial release probability. By measuring the rate of block of NMDA receptors by the open channel blocker MK-801, we confirmed that the initial release probability is greater at synapses onto interneurones versus pyramidal cells. This provides a mechanism by which both the initial strength and the short-term dynamics of Schaffer collateral excitatory synapses are regulated by their postsynaptic target cell.
Figures




References
-
- Augustin I, Rosenmund C, Südhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999;400:457–461. - PubMed
-
- Bellingham MC, Lim R, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

