Transcription coactivator peroxisome proliferator-activated receptor-binding protein/mediator 1 deficiency abrogates acetaminophen hepatotoxicity
- PMID: 16109766
- PMCID: PMC1187948
- DOI: 10.1073/pnas.0506000102
Transcription coactivator peroxisome proliferator-activated receptor-binding protein/mediator 1 deficiency abrogates acetaminophen hepatotoxicity
Abstract
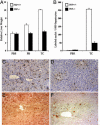
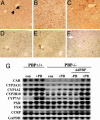
Peroxisome proliferator-activated receptor-binding protein (PBP), also known as thyroid hormone receptor-associated protein 220/vitamin D receptor-interacting protein 205/mediator 1, an anchor for multisubunit mediator transcription complex, functions as a transcription coactivator for nuclear receptors. Disruption of the PBP gene results in embryonic lethality around embryonic day 11.5 by affecting placental and multiorgan development. Here, we report that targeted deletion of PBP in liver parenchymal cells (PBP(Liv-/-)) results in the abrogation of hypertrophic and hyperplastic influences in liver mediated by constitutive androstane receptor (CAR) ligands phenobarbital (PB) and 1,4-bis-[2-(3,5-dichloropyridyloxy)]benzene, and of acetaminophen-induced hepatotoxicity. CAR interacts with the two nuclear receptor-interacting LXXLL (L, leucine; X, any amino acid) motifs in PBP in a ligand-dependent manner. We also show that PBP interacts with the C-terminal portion of CAR, suggesting that PBP is involved in the regulation of CAR function. Although the full-length PBP only minimally increased CAR transcriptional activity, a truncated form of PBP (amino acids 487-735) functioned as a dominant negative repressor, establishing that PBP functions as a coactivator for CAR. A reduction in CAR mRNA and protein level observed in PBP(Liv-/-) mouse liver suggests that PBP may regulate hepatic CAR expression. PBP-deficient hepatocytes in liver failed to reveal PB-dependent translocation of CAR to the nucleus. Adenoviral reconstitution of PBP in PBP(Liv-/-) mouse livers restored PB-mediated nuclear translocation of CAR as well as inducibility of CYP1A2, CYP2B10, CYP3A11, and CYP7A1 expression. We conclude that transcription coactivator PBP/TRAP220/MED1 is involved in the regulation of hepatic CAR function and that PBP deficiency in liver abrogates acetaminophen hepatotoxicity.
Figures


Similar articles
-
Transcription coactivator PRIP, the peroxisome proliferator-activated receptor (PPAR)-interacting protein, is redundant for the function of nuclear receptors PParalpha and CAR, the constitutive androstane receptor, in mouse liver.Gene Expr. 2007;13(4-5):255-69. doi: 10.3727/000000006780666948. Gene Expr. 2007. PMID: 17605299 Free PMC article.
-
Peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP) but not PPAR-interacting protein (PRIP) is required for nuclear translocation of constitutive androstane receptor in mouse liver.Biochem Biophys Res Commun. 2006 Aug 25;347(2):485-95. doi: 10.1016/j.bbrc.2006.06.129. Epub 2006 Jun 30. Biochem Biophys Res Commun. 2006. PMID: 16828057
-
Transcription coactivator PBP, the peroxisome proliferator-activated receptor (PPAR)-binding protein, is required for PPARalpha-regulated gene expression in liver.J Biol Chem. 2004 Jun 4;279(23):24427-34. doi: 10.1074/jbc.M402391200. Epub 2004 Mar 29. J Biol Chem. 2004. PMID: 15150259
-
Med1 subunit of the mediator complex in nuclear receptor-regulated energy metabolism, liver regeneration, and hepatocarcinogenesis.Gene Expr. 2014;16(2):63-75. doi: 10.3727/105221614X13919976902219. Gene Expr. 2014. PMID: 24801167 Free PMC article. Review.
-
Peroxisome proliferator-activated receptors, coactivators, and downstream targets.Cell Biochem Biophys. 2000;32 Spring:187-204. doi: 10.1385/cbb:32:1-3:187. Cell Biochem Biophys. 2000. PMID: 11330046 Review.
Cited by
-
Characterization of peroxisome proliferator-activated receptor alpha--independent effects of PPARalpha activators in the rodent liver: di-(2-ethylhexyl) phthalate also activates the constitutive-activated receptor.Toxicol Sci. 2010 Jan;113(1):45-59. doi: 10.1093/toxsci/kfp251. Epub 2009 Oct 22. Toxicol Sci. 2010. PMID: 19850644 Free PMC article.
-
Cardiac Med1 deletion promotes early lethality, cardiac remodeling, and transcriptional reprogramming.Am J Physiol Heart Circ Physiol. 2017 Apr 1;312(4):H768-H780. doi: 10.1152/ajpheart.00728.2016. Epub 2017 Feb 3. Am J Physiol Heart Circ Physiol. 2017. PMID: 28159809 Free PMC article.
-
Nuclear receptor coactivator 6 mediates the synergistic activation of human cytochrome P-450 2C9 by the constitutive androstane receptor and hepatic nuclear factor-4alpha.Mol Pharmacol. 2008 Sep;74(3):913-23. doi: 10.1124/mol.108.048983. Epub 2008 Jun 13. Mol Pharmacol. 2008. PMID: 18552123 Free PMC article.
-
An integrative genomic analysis identifies Bhmt2 as a diet-dependent genetic factor protecting against acetaminophen-induced liver toxicity.Genome Res. 2010 Jan;20(1):28-35. doi: 10.1101/gr.097212.109. Epub 2009 Nov 18. Genome Res. 2010. PMID: 19923254 Free PMC article.
-
Early embryonic lethality of mice with disrupted transcription cofactor PIMT/NCOA6IP/Tgs1 gene.Mech Dev. 2012 Sep-Dec;129(9-12):193-207. doi: 10.1016/j.mod.2012.08.002. Epub 2012 Sep 7. Mech Dev. 2012. PMID: 22982455 Free PMC article.
References
-
- Glass C. K. & Rosenfeld, M. G. (2000) Genes Dev. 14, 121-141. - PubMed
-
- McKenna, N. J. & O'Malley, B. W. (2002) Cell 108, 465-474. - PubMed
-
- Lewis, B. A. & Reinberg, D. (2003) J. Cell Sci. 116, 3667-3675. - PubMed
-
- Zhu, Y., Qi, C., Jain, S., Rao, M. S. & Reddy, J. K. (1997) J. Biol. Chem. 272, 25500-25506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

