Cytochrome c maturation and the physiological role of c-type cytochromes in Vibrio cholerae
- PMID: 16109941
- PMCID: PMC1196146
- DOI: 10.1128/JB.187.17.5996-6004.2005
Cytochrome c maturation and the physiological role of c-type cytochromes in Vibrio cholerae
Abstract
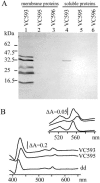
Vibrio cholerae lives in different habitats, varying from aquatic ecosystems to the human intestinal tract. The organism has acquired a set of electron transport pathways for aerobic and anaerobic respiration that enable adaptation to the various environmental conditions. We have inactivated the V. cholerae ccmE gene, which is required for cytochrome c biogenesis. The resulting strain is deficient of all c-type cytochromes and allows us to characterize the physiological role of these proteins. Under aerobic conditions in rich medium, V. cholerae produces at least six c-type cytochromes, none of which is required for growth. Wild-type V. cholerae produces active fumarate reductase, trimethylamine N-oxide reductase, cbb3 oxidase, and nitrate reductase, of which only the fumarate reductase does not require maturation of c-type cytochromes. The reduction of nitrate in the medium resulted in the accumulation of nitrite, which is toxic for the cells. This suggests that V. cholerae is able to scavenge nitrate from the environment only in the presence of other nitrite-reducing organisms. The phenotypes of cytochrome c-deficient V. cholerae were used in a transposon mutagenesis screening to search for additional genes required for cytochrome c maturation. Over 55,000 mutants were analyzed for nitrate reductase and cbb3 oxidase activity. No transposon insertions other than those within the ccm genes for cytochrome c maturation and the dsbD gene, which encodes a disulphide bond reductase, were found. In addition, the role of a novel CcdA-like protein in cbb3 oxidase assembly is discussed.
Figures


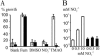

References
-
- Arslan, E., H. Schulz, R. Zufferey, P. Künzler, and L. Thöny-Meyer. 1998. Overproduction of the Bradyrhizobium japonicum c-type cytochrome subunits of the cbb3 oxidase in Escherichia coli. Biochem. Biophys. Res. Commun. 251:744-747. - PubMed
-
- Braun, M., I. García Rubio, and L. Thöny-Meyer. 2005. A heam tag for in vivo synthesis of artificial cytochromes. Appl. Microbiol. Biotechnol. 67:234-239. - PubMed
-
- Braun, M., and T. J. Silhavy. 2002. Imp/OstA is required for cell envelope biogenesis in Escherichia coli. Mol. Microbiol. 45:1289-1302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

