TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury
- PMID: 16110328
- PMCID: PMC1187934
- DOI: 10.1172/JCI25437
TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury
Erratum in
- J Clin Invest. 2010 Jan;120(1):394
Abstract
Cold hyperalgesia is a well-documented symptom of inflammatory and neuropathic pain; however, the underlying mechanisms of this enhanced sensitivity to cold are poorly understood. A subset of transient receptor potential (TRP) channels mediates thermosensation and is expressed in sensory tissues, such as nociceptors and skin. Here we report that the pharmacological blockade of TRPA1 in primary sensory neurons reversed cold hyperalgesia caused by inflammation and nerve injury. Inflammation and nerve injury increased TRPA1, but not TRPM8, expression in tyrosine kinase A-expressing dorsal root ganglion (DRG) neurons. Intrathecal administration of anti-nerve growth factor (anti-NGF), p38 MAPK inhibitor, or TRPA1 antisense oligodeoxynucleotide decreased the induction of TRPA1 and suppressed inflammation- and nerve injury-induced cold hyperalgesia. Conversely, intrathecal injection of NGF, but not glial cell line-derived neurotrophic factor, increased TRPA1 in DRG neurons through the p38 MAPK pathway. Together, these results demonstrate that an NGF-induced TRPA1 increase in sensory neurons via p38 activation is necessary for cold hyperalgesia. Thus, blocking TRPA1 in sensory neurons might provide a fruitful strategy for treating cold hyperalgesia caused by inflammation and nerve damage.
Figures
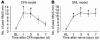


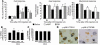
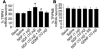

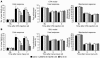

References
-
- LaMotte RH, Thalhammer JG. Response properties of high-threshold cutaneous cold receptors in the primate. Brain Res. 1982;244:279–287. - PubMed
-
- Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J. Neurophysiol. 1992;68:581–595. - PubMed
-
- Hao JX, Yu W, Xu XJ, Wiesenfeld-Hallin Z. Capsaicin-sensitive afferents mediate chronic cold, but not mechanical, allodynia-like behavior in spinally injured rats. Brain Res. 1996;722:177–180. - PubMed
-
- Hama AT. Capsaicin-sensitive primary afferents mediate responses to cold in rats with a peripheral mononeuropathy. Neuroreport. 2002;13:461–464. - PubMed
-
- Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr. Opin. Neurobiol. 2003;13:487–492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

