Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala
- PMID: 16111818
- PMCID: PMC2367315
- DOI: 10.1016/j.neuroscience.2005.06.038
Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala
Abstract
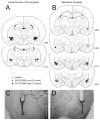
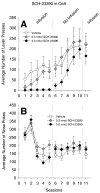
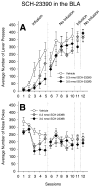
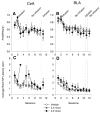
Substantial experimental evidence exists suggesting a critical role for dopamine in reinforcer-related processes, such as learning and drug addiction. Dopamine receptors, and in particular D1 receptors, are widely considered as modulators of synaptic plasticity. The amygdala contains both dopamine terminals and dopamine D1 receptors and is intimately involved in motivation and learning. However, little is known about the involvement of D1 receptor activation in two subnuclei of the mammalian amygdala, the central nucleus and basolateral complex in instrumental learning. Following recovery from surgery and preliminary training, rats with bilateral indwelling cannulae aimed at the central nucleus or basolateral complex were trained to lever-press for sucrose pellets over 12 sessions. Infusion of the selective D1 antagonist R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (0.3 nmol and 3.0 nmol) prior to the first five training sessions dose-dependently impaired instrumental learning when compared with vehicle-infused controls. All rats were then exposed to five sessions drug-free; lever-pressing quickly reached equal levels across groups. A drug infusion prior to an 11th session revealed no effect on performance. Control experiments indicated that basic motivational processes and general motor responses were intact, such as spontaneous feeding and locomotor activity. These results show an essential role for D1-receptor activation in both the central nucleus and basolateral complex on the acquisition of lever pressing for sucrose pellets in rats, but not the performance of the behavior once conditioned. We propose that instrumental learning is dependent on plasticity in the central nucleus and basolateral complex amygdala, and that D1 receptor activation participates in transcriptional processes that underlie this plasticity.
Figures
Similar articles
-
Dissociating ventral and dorsal subicular dopamine D1 receptor involvement in instrumental learning, spontaneous motor behavior, and motivation.Behav Neurosci. 2006 Jun;120(3):542-53. doi: 10.1037/0735-7044.120.3.542. Behav Neurosci. 2006. PMID: 16768606 Free PMC article.
-
Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex.J Neurosci. 2002 Feb 1;22(3):1063-71. doi: 10.1523/JNEUROSCI.22-03-01063.2002. J Neurosci. 2002. PMID: 11826135 Free PMC article.
-
Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior.Psychopharmacology (Berl). 2001 Mar;154(3):301-10. doi: 10.1007/s002130000636. Psychopharmacology (Berl). 2001. PMID: 11351937
-
Dissociable hippocampal and amygdalar D1-like receptor contribution to discriminated Pavlovian conditioned approach learning.Behav Brain Res. 2016 Feb 15;299:111-21. doi: 10.1016/j.bbr.2015.11.034. Epub 2015 Nov 26. Behav Brain Res. 2016. PMID: 26632336 Free PMC article.
-
Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning.J Neurosci. 2000 Oct 15;20(20):7737-42. doi: 10.1523/JNEUROSCI.20-20-07737.2000. J Neurosci. 2000. PMID: 11027236 Free PMC article.
Cited by
-
Involvement of Basolateral Amygdala Dopamine D1 Receptors in the Acquisition and Expression of Morphine-Induced Place Preference in Rats.Adv Biomed Res. 2022 Jan 31;11:8. doi: 10.4103/abr.abr_284_21. eCollection 2022. Adv Biomed Res. 2022. PMID: 35284350 Free PMC article.
-
Modulation of risk/reward decision making by dopaminergic transmission within the basolateral amygdala.Psychopharmacology (Berl). 2016 Jan;233(1):121-36. doi: 10.1007/s00213-015-4094-8. Epub 2015 Oct 3. Psychopharmacology (Berl). 2016. PMID: 26432096
-
The clinical relevance of neuroplasticity in corticostriatal networks during operant learning.Neurosci Biobehav Rev. 2013 Nov;37(9 Pt A):2071-80. doi: 10.1016/j.neubiorev.2013.03.019. Epub 2013 Apr 5. Neurosci Biobehav Rev. 2013. PMID: 23567518 Free PMC article. Review.
-
Alcohol and cannabinoid binges and daily exposure to nicotine in adolescent/young adult rats induce sex-dependent long-term appetitive instrumental learning impairment.Front Behav Neurosci. 2023 Feb 6;17:1129866. doi: 10.3389/fnbeh.2023.1129866. eCollection 2023. Front Behav Neurosci. 2023. PMID: 36815183 Free PMC article.
-
Methylphenidate facilitates learning-induced amygdala plasticity.Nat Neurosci. 2010 Apr;13(4):475-81. doi: 10.1038/nn.2506. Epub 2010 Mar 7. Nat Neurosci. 2010. PMID: 20208527 Free PMC article.
References
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11(2):180–187. - PubMed
-
- Abercrombie ED, Bonatz AE, Zigmond MJ. Effects of L-dopa on extracellular dopamine in striatum of normal and 6-hydroxydopamine-treated rats. Brain Res. 1990;525(1):36–44. - PubMed
-
- Asan E. Ultrastructural features of tyrosine-hydroxylase-immunoreactive afferents and their targets in the rat amygdala. Cell Tissue Res. 1997;288(3):449–469. - PubMed
-
- Baldwin AE, Holahan MR, Sadeghian K, Kelley AE. N-methyl-D-aspartate receptor-dependent plasticity within a distributed corticostriatal network mediates appetitive instrumental learning. Behav Neurosci. 2000;114(1):84–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

