N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein
- PMID: 16113116
- PMCID: PMC1366831
- DOI: 10.1529/biophysj.105.068759
N-terminal-mediated homomultimerization of prestin, the outer hair cell motor protein
Abstract
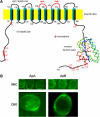
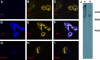
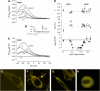
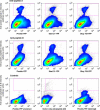
The outer hair cell lateral membrane motor, prestin, drives the cell's mechanical response that underpins mammalian cochlear amplification. Little is known about the protein's structure-function relations. Here we provide evidence that prestin is a 10-transmembrane domain protein whose membrane topology differs from that of previous models. We also present evidence that both intracellular termini of prestin are required for normal voltage sensing, with short truncations of either terminal resulting in absent or modified activity despite quantitative findings of normal membrane targeting. Finally, we show with fluorescence resonance energy transfer that prestin-prestin interactions are dependent on an intact N-terminus, suggesting that this terminus is important for homo-oligomerization of prestin. These domains, which we have perturbed, likely contribute to allosteric modulation of prestin via interactions among prestin molecules or possibly between prestin and other proteins, as well.
Figures

References
-
- Zheng, J., W. Shen, D. Z. He, K. B. Long, L. D. Madison, and P. Dallos. 2000. Prestin is the motor protein of cochlear outer hair cells. Nature. 405:149–155. - PubMed
-
- Liberman, M. C., J. Gao, D. Z. He, X. Wu, S. Jia, and J. Zuo. 2002. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 419:300–304. - PubMed
-
- Meltzer, J., and J. Santos-Sacchi. 2001. Temperature dependence of non-linear capacitance in human embryonic kidney cells transfected with prestin, the outer hair cell motor protein. Neurosci. Lett. 313:141–144. - PubMed
-
- Santos-Sacchi, J., and E. Navarrete. 2002. Voltage-dependent changes in specific membrane capacitance caused by prestin, the outer hair cell lateral membrane motor. Pflugers Arch. 444:99–106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

