Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease
- PMID: 16113133
- PMCID: PMC1720585
- DOI: 10.1136/adc.2004.068890
Acid suppression does not change respiratory symptoms in children with asthma and gastro-oesophageal reflux disease
Abstract
Background: Epidemiological studies have shown an association between gastro-oesophageal reflux disease (GORD) and asthma, and oesophageal acid perfusion may cause bronchial constriction. However, no causative relation has been proven.
Aim: To assess whether acid suppression would lead to reduced asthma symptoms in children with concomitant asthma and GORD.
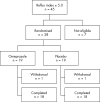
Methods: Thirty eight children (mean age 10.8 years, range 7.2-16.8; 29 males) with asthma and a reflux index > or =5.0 assessed by 24 hour oesophageal pH monitoring were randomised to 12 weeks of treatment with omeprazole 20 mg daily or placebo. The groups were similar in age, gender, mean reflux index, and asthma severity. Primary endpoints were asthma symptoms (daytime wheeze, symptoms at night, in the morning, and during exercise) and quality of life (PAQLQ). Secondary endpoints were changes in lung function and the use of short acting bronchodilators. At the end of the study a repeated pH study was performed to confirm the efficacy of acid suppression.
Results: The change in total symptom score did not differ significantly between the omeprazole and the placebo group, and decreased by 1.28 (95% CI -0.1 to 2.65) and 1.28 (95% CI -0.72 to 3.27) respectively. The PAQLQ score increased by 0.62 (95% CI 0.29 to 0.95) in the omeprazole group compared to 0.50 (95% CI 0.29 to 0.70) in the placebo group. Change in lung function and use of short acting bronchodilators were similar in the groups. The acid suppression was adequate (reflux index <5.0) under omeprazole treatment.
Conclusion: Omeprazole treatment did not improve asthma symptoms or lung function in children with asthma and GORD.