Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses
- PMID: 16113213
- PMCID: PMC1203376
- DOI: 10.1104/pp.105.065698
Enhancing Arabidopsis salt and drought stress tolerance by chemical priming for its abscisic acid responses
Abstract
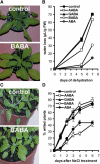
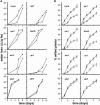
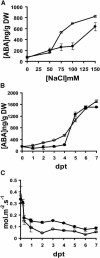
Drought and salt stress tolerance of Arabidopsis (Arabidopsis thaliana) plants increased following treatment with the nonprotein amino acid beta-aminobutyric acid (BABA), known as an inducer of resistance against infection of plants by numerous pathogens. BABA-pretreated plants showed earlier and higher expression of the salicylic acid-dependent PR-1 and PR-5 and the abscisic acid (ABA)-dependent RAB-18 and RD-29A genes following salt and drought stress. However, non-expressor of pathogenesis-related genes 1 and constitutive expressor of pathogenesis-related genes 1 mutants as well as transgenic NahG plants, all affected in the salicylic acid signal transduction pathway, still showed increased salt and drought tolerance after BABA treatment. On the contrary, the ABA deficient 1 and ABA insensitive 4 mutants, both impaired in the ABA-signaling pathway, could not be protected by BABA application. Our data demonstrate that BABA-induced water stress tolerance is based on enhanced ABA accumulation resulting in accelerated stress gene expression and stomatal closure. Here, we show a possibility to increase plant tolerance for these abiotic stresses through effective priming of the preexisting defense pathways without resorting to genetic alterations.
Figures

References
-
- Amzallag GN, Lerner HR, Poljakoff-Mayber A (1990) Exogenous ABA as a modulator of the response of sorghum to high salinity. J Exp Bot 41: 1529–1534
-
- Bates LS, Waldre RP, Teare ID (1973) Rapid determination of free proline for water stress studies. Plant Soil 39: 205–208
-
- Blatt MR (2000) Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol 16: 221–241 - PubMed
-
- Bray EA (1997) Plant responses to water deficit. Trends Plant Sci 2: 48–54
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

