Role of FliF and FliI of Listeria monocytogenes in flagellar assembly and pathogenicity
- PMID: 16113269
- PMCID: PMC1231047
- DOI: 10.1128/IAI.73.9.5530-5539.2005
Role of FliF and FliI of Listeria monocytogenes in flagellar assembly and pathogenicity
Erratum in
- Infect Immun. 2007 Feb;75(2):1063-4
Abstract
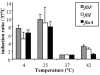
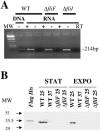
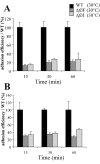
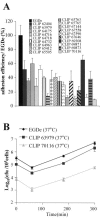
Flagellar structures have been shown to participate in virulence in a variety of intestinal pathogens. Here, we have identified two potential flagellar genes of Listeria monocytogenes: lmo0713, encoding a protein similar to the flagellar basal body component FliF, and lmo0716, encoding a protein similar to FliI, the cognate ATPase energizing the flagellar export apparatus. Expression of fliF and fliI appears to be downregulated at 37 degrees C, like that of flaA, encoding flagellin. By constructing two chromosomal deletion mutants, we show that inactivation of either fliF or fliI (i) abolishes bacterial motility and flagella production, (ii) impairs adhesion and entry into nonphagocytic epithelial cells, and (iii) also reduces uptake by bone marrow-derived macrophages. However, the DeltafliF and DeltafliI mutations have only a minor impact on bacterial virulence in the mouse model, indicating that the flagellar secretion apparatus itself is not essential for survival in this animal model. Finally, among 100 human clinical isolates of L. monocytogenes tested, we found 20 strains still motile at 37 degrees C. Notably, all these strains adhered less efficiently than strain EGD-e to Caco-2 cells at 37 degrees C but showed no defect of intracellular multiplication. These data suggest that expression of the flagella at 37 degrees C might hinder optimal adhesion to epithelial cells but has no impact on intracytosolic survival of L. monocytogenes.
Figures
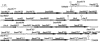


References
-
- Bonnemain, C., C. Raynaud, H. Reglier-Poupet, I. Dubail, C. Frehel, M. A. Lety, P. Berche, and A. Charbit. 2004. Differential roles of multiple signal peptidases in the virulence of Listeria monocytogenes. Mol. Microbiol. 51:1251-1266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

