Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis
- PMID: 16113301
- PMCID: PMC1231065
- DOI: 10.1128/IAI.73.9.5827-5834.2005
Toward a novel experimental model of infection to study American cutaneous leishmaniasis caused by Leishmania braziliensis
Abstract
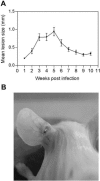
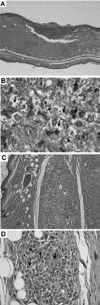
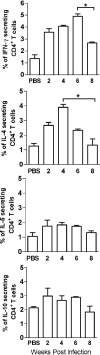
Leishmania spp. cause a broad spectrum of diseases collectively known as leishmaniasis. Leishmania braziliensis is the main etiological agent of American cutaneous leishmaniasis (ACL) and mucocutaneous leishmaniasis. In the present study, we have developed an experimental model of infection that closely resembles ACL caused by L. braziliensis. In order to do so, BALB/c mice were infected in the ear dermis with 10(5) parasites and distinct aspects of the infection were evaluated. Following inoculation, parasite expansion in the ear dermis was accompanied by the development of an ulcerated dermal lesion which healed spontaneously, as seen by the presence of a scar. Histological analysis of infected ears showed the presence of a mixed inflammatory infiltrate consisting of both mononuclear and polymorphonuclear cells. In draining lymph nodes, parasite replication was detected throughout the infection. In vitro restimulation of draining lymph node cells followed by intracellular staining showed an up-regulation in the production of gamma interferon (IFN-gamma) and in the frequency of IFN-gamma-secreting CD4(+) and CD8(+) T cells. Reverse transcription-PCR of ears and draining lymph node cells showed the expression of CC chemokines. The dermal model of infection with L. braziliensis herein is able to reproduce aspects of the natural infection, such as the presence of an ulcerated lesion, parasite dissemination to lymphoid areas, and the development of a Th1-type immune response. These results indicate that this model shall be useful to address questions related to the concomitant immunity to reinfection and parasite persistence leading to mucocutaneous leishmaniasis.
Figures


Similar articles
-
The presence of Tregs does not preclude immunity to reinfection with Leishmania braziliensis.Int J Parasitol. 2012 Jul;42(8):771-80. doi: 10.1016/j.ijpara.2012.05.006. Epub 2012 Jun 13. Int J Parasitol. 2012. PMID: 22705204
-
Testing of four Leishmania vaccine candidates in a mouse model of infection with Leishmania (Viannia) braziliensis, the main causative agent of cutaneous leishmaniasis in the New World.Clin Vaccine Immunol. 2007 Sep;14(9):1173-81. doi: 10.1128/CVI.00060-07. Epub 2007 Jul 11. Clin Vaccine Immunol. 2007. PMID: 17626159 Free PMC article.
-
Immunopathological characterization of human cutaneous leishmaniasis lesions caused by Leishmania (Viannia) spp. in Amazonian Brazil.Parasitol Res. 2017 May;116(5):1423-1431. doi: 10.1007/s00436-017-5403-4. Epub 2017 Feb 21. Parasitol Res. 2017. PMID: 28224222
-
Distinct immunological states in murine cutaneous leishmaniasis by immunising with different amounts of antigen: the generation of beneficial, potentially harmful, harmful and potentially extremely harmful states.Behring Inst Mitt. 1997 Feb;(98):153-9. Behring Inst Mitt. 1997. PMID: 9382736 Review.
-
Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis.Parasite Immunol. 2009 Aug;31(8):423-31. doi: 10.1111/j.1365-3024.2009.01116.x. Parasite Immunol. 2009. PMID: 19646206 Review.
Cited by
-
Cutaneous leishmaniasis: immune responses in protection and pathogenesis.Nat Rev Immunol. 2016 Sep;16(9):581-92. doi: 10.1038/nri.2016.72. Epub 2016 Jul 18. Nat Rev Immunol. 2016. PMID: 27424773 Review.
-
The effect of (-)-epigallocatechin 3-O--gallate in vitro and in vivo in Leishmania braziliensis: involvement of reactive oxygen species as a mechanism of action.PLoS Negl Trop Dis. 2014 Aug 21;8(8):e3093. doi: 10.1371/journal.pntd.0003093. eCollection 2014 Aug. PLoS Negl Trop Dis. 2014. PMID: 25144225 Free PMC article.
-
Host genetic factors in American cutaneous leishmaniasis: a critical appraisal of studies conducted in an endemic area of Brazil.Mem Inst Oswaldo Cruz. 2014 Jun;109(3):279-88. doi: 10.1590/0074-0276140028. Epub 2014 May 27. Mem Inst Oswaldo Cruz. 2014. PMID: 24863979 Free PMC article. Review.
-
Comparative evaluation of lesion development, tissue damage, and cytokine expression in golden hamsters (Mesocricetus auratus) infected by inocula with different Leishmania (Viannia) braziliensis concentrations.Infect Immun. 2014 Dec;82(12):5203-13. doi: 10.1128/IAI.02083-14. Epub 2014 Oct 6. Infect Immun. 2014. PMID: 25287925 Free PMC article.
-
Evaluation of in vitro and in vivo Efficacy of a Novel Amphotericin B-Loaded Nanostructured Lipid Carrier in the Treatment of Leishmania braziliensis Infection.Int J Nanomedicine. 2020 Nov 5;15:8659-8672. doi: 10.2147/IJN.S262642. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33177824 Free PMC article.
References
-
- Barral, A., M. Barral-Netto, R. Almeida, A. R. de Jesus, G. Grimaldi Junior, E. M. Netto, I. Santos, O. Bacellar, and E. M. Carvalho. 1992. Lymphadenopathy associated with Leishmania braziliensis cutaneous infection. Am. J. Trop. Med. Hyg. 47:587-592. - PubMed
-
- Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

