Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice
- PMID: 16113303
- PMCID: PMC1231080
- DOI: 10.1128/IAI.73.9.5842-5852.2005
Vaccination with the Leishmania infantum acidic ribosomal P0 protein plus CpG oligodeoxynucleotides induces protection against cutaneous leishmaniasis in C57BL/6 mice but does not prevent progressive disease in BALB/c mice
Abstract
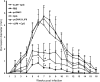
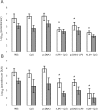
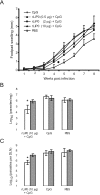
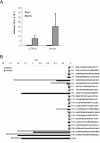
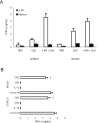
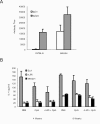
We have examined the efficacy of the administration in mice of a molecularly defined vaccine based on the Leishmania infantum acidic ribosomal protein P0 (rLiP0). Two different challenge models of murine cutaneous leishmaniasis were used: (i) subcutaneous inoculation of L. major parasites in susceptible BALB/c mice (a model widely used for vaccination analysis) and (ii) the intradermal inoculation of a low infective dose in resistant C57BL/6 mice (a model that more accurately reproduces the L. major infection in natural reservoirs and in human hosts). First, we demonstrated that C57BL/6 mice vaccinated with LiP0-DNA or rLiP0 protein plus CpG oligodeoxynucleotides (ODN) were protected against the development of dermal pathology and showed a reduction in the parasite load. This protection was associated with production of gamma interferon (IFN-gamma) in the dermal site. Secondly, we showed that immunization with rLiP0 plus CpG ODN is able to induce only partial protection in BALB/c, since these mice finally developed a progressive disease. Further, we demonstrated that LiP0 vaccination induces a Th1 immunological response in both strains of mice. In both cases, the antibodies against LiP0 were predominantly of the immunoglobulin G2a isotype, which was correlated with an rLiP0-stimulated production of IFN-gamma in draining lymph nodes. Finally, we demonstrated that LiP0 vaccination does not prevent the Th2 response induced by L. major infection in BALB/c mice. Taken together, these data indicate that the BALB/c model of cutaneous leishmaniasis may undervalue the potential efficacy of some vaccines based on defined proteins, making C57BL/6 a suitable alternative model to test vaccine candidates.
Figures


References
-
- Belkaid, Y., S. Kamhawi, G. Modi, J. Valenzuela, N. Noben-Trauth, E. Rowton, J. Ribeiro, and D. L. Sacks. 1998. Development of a natural model of cutaneous leishmaniasis: powerful effects of vector saliva and saliva preexposure on the long-term outcome of Leishmania major infection in the mouse ear dermis. J. Exp. Med. 188:1941-1953. - PMC - PubMed
-
- Belkaid, Y., E. Von Stebut, S. Mendez, R. Lira, E. Caler, S. Bertholet, M. C. Udey, and D. Sacks. 2002. CD8+ T cells are required for primary immunity in C57BL/6 mice following low-dose, intradermal challenge with Leishmania major. J. Immunol. 168:3992-4000. - PubMed
-
- Cho, H. J., K. Takabayashi, P. M. Cheng, M. D. Nguyen, M. Corr, S. Tuck, and E. Raz. 2000. Immunostimulatory DNA-based vaccines induce cytotoxic lymphocyte activity by a T-helper cell-independent mechanism. Nat. Biotechnol. 18:509-514. - PubMed
-
- Coffman, R. L. 1993. Mechanisms of helper T-cell regulation of B-cell activity. Ann. N. Y. Acad. Sci. 681:25-28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

