Cyclic diguanylate regulates Vibrio cholerae virulence gene expression
- PMID: 16113306
- PMCID: PMC1231145
- DOI: 10.1128/IAI.73.9.5873-5882.2005
Cyclic diguanylate regulates Vibrio cholerae virulence gene expression
Abstract
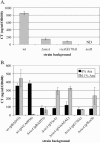
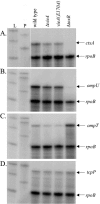
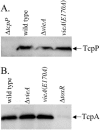
The cyclic dinucleotide second messenger cyclic diguanylate (c-diGMP) has been implicated in regulation of cell surface properties in several bacterial species, including Vibrio cholerae. Expression of genes required for V. cholerae biofilm formation is activated by an increased intracellular c-diGMP concentration. The response regulator VieA, which contains a domain responsible for degradation of c-diGMP, is required to maintain a low concentration of c-diGMP and repress biofilm formation. The VieSAB three-component signal transduction system was, however, originally identified as a regulator of ctxAB, the genes encoding cholera toxin (CT). Here we show that the c-diGMP phosphodiesterase activity of VieA is required to enhance CT production. This regulation occurred at the transcriptional level, and ectopically altering the c-diGMP concentration by expression of diguanylate cyclase or phosphodiesterase enzymes also affected ctxAB transcription. The c-diGMP phosphodiesterase activity of VieA was also required for maximal transcription toxT but did not influence the activity of ToxR or expression of TcpP. Finally, a single amino acid substitution in VieA that increases the intracellular c-diGMP concentration led to attenuation in the infant mouse model of cholera. Since virulence genes including toxT and ctxA are repressed by a high concentration of c-diGMP, while biofilm genes are activated, we suggest that c-diGMP signaling is important for the transition of V. cholerae from the environment to the host.
Figures




References
-
- Brown, R. C., and R. K. Taylor. 1995. Organization of tcp, acf, and toxT genes within a ToxT-dependent operon. Mol. Microbiol. 16:425-439. - PubMed
-
- Champion, G. A., M. N. Neely, M. A. Brennan, and V. J. DiRita. 1997. A branch in the ToxR regulatory cascade of Vibrio cholerae revealed by characterization of toxT mutant strains. Mol. Microbiol. 23:323-331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

