Merozoite surface protein 1 of Plasmodium vivax induces a protective response against Plasmodium cynomolgi challenge in rhesus monkeys
- PMID: 16113314
- PMCID: PMC1231099
- DOI: 10.1128/IAI.73.9.5936-5944.2005
Merozoite surface protein 1 of Plasmodium vivax induces a protective response against Plasmodium cynomolgi challenge in rhesus monkeys
Abstract
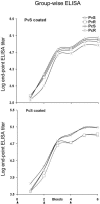
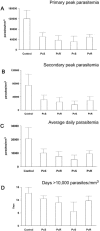
The 42-kDa fragment of the merozoite surface protein 1 (MSP-1(42)) is a leading candidate for the development of a vaccine to control malaria. We previously reported a method for the production of Plasmodium vivax MSP-1(42) (PvMSP-1(42)) as a soluble protein (S. Dutta, L. W. Ware, A. Barbosa, C. F. Ockenhouse, and D. E. Lanar, Infect. Immun. 69:5464-5470, 2001). We report here a process to manufacture the same PvMSP-1(42) protein but as an insoluble inclusion body-derived protein which was then refolded in vitro. We compared the immunogenicity and protective efficacy of the soluble and refolded forms of PvMSP-1(42) protein by using a heterologous but closely related P. cynomolgi-rhesus monkey challenge model. As comparative controls we also expressed, purified, and immunized rhesus with the soluble and refolded forms of the P. cynomolgi MSP-1(42) (PcMSP-1(42)) proteins. All proteins induced equally high-titer, cross-reacting antibodies. Upon challenge with P. cynomolgi, none of the MSP-1(42)-vaccinated groups demonstrated sterile protection or a delay in the prepatent period. However, following an initial rise in parasitemia, all MSP-1-vaccinated animals had significantly lower parasite burdens as indicated by lower cumulative parasitemia, lower peak parasitemia, lower secondary peak parasitemia, and lower average daily parasitemia compared to the adjuvant control group (P < 0.05). Except the soluble PcMSP-1(42) group, monkeys in all other groups had fewer numbers of days with parasitemia of >10,000 parasites mm(-3). Interestingly, there was no significant difference in the level of partial protection observed in the homologous and heterologous groups in this challenge model. The soluble and refolded forms of PcMSP-1(42) and PvMSP-1(42) proteins also appeared to have a similar partially protective effect.
Figures


Similar articles
-
Biochemical and immunological characterization of E. coli expressed 42 kDa fragment of Plasmodium vivax and P. cynomolgi bastianelli merozoite surface protein-1.Indian J Biochem Biophys. 2007 Dec;44(6):429-36. Indian J Biochem Biophys. 2007. PMID: 18320841
-
Immunogenicity of a plasmid DNA vaccine encoding 42kDa fragment of Plasmodium vivax merozoite surface protein-1.Acta Trop. 2016 Oct;162:66-74. doi: 10.1016/j.actatropica.2016.06.013. Epub 2016 Jun 14. Acta Trop. 2016. PMID: 27311385
-
Plasmodium vivax, P. cynomolgi, and P. knowlesi: identification of homologue proteins associated with the surface of merozoites.Exp Parasitol. 1999 Mar;91(3):238-49. doi: 10.1006/expr.1998.4372. Exp Parasitol. 1999. PMID: 10072326
-
N-terminal Plasmodium vivax merozoite surface protein-1, a potential subunit for malaria vivax vaccine.Clin Dev Immunol. 2013;2013:965841. doi: 10.1155/2013/965841. Epub 2013 Sep 28. Clin Dev Immunol. 2013. PMID: 24187566 Free PMC article. Review.
-
The New Zoonotic Malaria: Plasmodium cynomolgi.Trop Med Infect Dis. 2021 Apr 5;6(2):46. doi: 10.3390/tropicalmed6020046. Trop Med Infect Dis. 2021. PMID: 33916448 Free PMC article. Review.
Cited by
-
Plasmodium vivax: who cares?Malar J. 2008 Dec 11;7 Suppl 1(Suppl 1):S9. doi: 10.1186/1475-2875-7-S1-S9. Malar J. 2008. PMID: 19091043 Free PMC article. Review.
-
Preclinical assessment of the receptor-binding domain of Plasmodium vivax Duffy-binding protein as a vaccine candidate in rhesus macaques.Vaccine. 2008 Aug 12;26(34):4338-44. doi: 10.1016/j.vaccine.2008.06.010. Epub 2008 Jun 23. Vaccine. 2008. PMID: 18573299 Free PMC article.
-
Evaluation of recombinant Plasmodium knowlesi merozoite surface protein-1(33) for detection of human malaria.Am J Trop Med Hyg. 2013 May;88(5):835-40. doi: 10.4269/ajtmh.12-0250. Epub 2013 Mar 18. Am J Trop Med Hyg. 2013. PMID: 23509118 Free PMC article.
-
Immunogenicity of bacterial-expressed recombinant Plasmodium knowlesi merozoite surface protein-142 (MSP-142).Malar J. 2013 Dec 19;12:454. doi: 10.1186/1475-2875-12-454. Malar J. 2013. PMID: 24354660 Free PMC article.
-
Genetic polymorphism and natural selection in the C-terminal 42 kDa region of merozoite surface protein-1 among Plasmodium vivax Korean isolates.Malar J. 2012 Jun 18;11:206. doi: 10.1186/1475-2875-11-206. Malar J. 2012. PMID: 22709605 Free PMC article.
References
-
- Ahlborg, N., I. T. Ling, W. Howard, A. A. Holder, and E. M. Riley. 2002. Protective immune responses to the 42-kilodalton (kDa) region of Plasmodium yoelii merozoite surface protein 1 are induced by the C-terminal 19-kDa region but not by the adjacent 33-kDa region. Infect. Immun. 70:820-825. - PMC - PubMed
-
- Angov, E., B. M. Aufiero, A. M. Turgeon, M. Van Handenhove, C. F. Ockenhouse, K. E. Kester, D. S. Walsh, J. S. McBride, M. C. Dubois, J. Cohen, J. D. Haynes, K. H. Eckels, D. G. Heppner, W. R. Ballou, C. L. Diggs, and J. A. Lyon. 2003. Development and pre-clinical analysis of a Plasmodium falciparum merozoite surface protein-1(42) malaria vaccine. Mol. Biochem. Parasitol. 128:195-204. - PubMed
-
- Ballou, W. R., M. Arevalo-Herrera, D. Carucci, T. L. Richie, G. Corradin, C. L. Diggs, P. Druilhe, B. K. Giersing, A. Saul, D. G. Heppner, K. E. Kester, D. E. Lanar, J. Lyon, A. V. Hill, W. Pan, and J. D. Cohen. 2004. Update on the clinical development of candidate malaria vaccines. Am. J. Trop. Med. Hyg. 71(Suppl. 2):239-247. - PubMed
-
- Benjamin, P. A., I. T. Ling, G. Clottey, L. M. Valero, S. A. Ogun, S. L. Fleck, D. Walliker, W. D. Morgan, B. Birdsall, J. Feeney, and A. A. Holder. 1999. Antigenic and sequence diversity at the C-terminus of the merozoite surface protein-1 from rodent malaria isolates, and the binding of protective monoclonal antibodies. Mol. Biochem. Parasitol. 104:147-156. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

