CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2
- PMID: 16113322
- PMCID: PMC1231112
- DOI: 10.1128/IAI.73.9.6017-6025.2005
CD8+-T-cell-dependent control of Trypanosoma cruzi infection in a highly susceptible mouse strain after immunization with recombinant proteins based on amastigote surface protein 2
Abstract
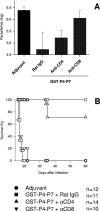
We previously described that DNA vaccination with the gene encoding amastigote surface protein 2 (ASP-2) protects approximately 65% of highly susceptible A/Sn mice against the lethal Trypanosoma cruzi infection. Here, we explored the possibility that bacterial recombinant proteins of ASP-2 could be used to improve the efficacy of vaccinations. Initially, we compared the protective efficacy of vaccination regimens using either a plasmid DNA, a recombinant protein, or both sequentially (DNA priming and protein boosting). Survival after the challenge was not statistically different among the three mouse groups and ranged from 53.5 to 75%. The fact that immunization with a recombinant protein alone induced protective immunity revealed the possibility that this strategy could be pursued for vaccination. We investigated this possibility by using six different recombinant proteins representing distinct portions of ASP-2. The vaccination of mice with glutathione S-transferase fusion proteins representing amino acids 261 to 500 or 261 to 380 of ASP-2 in the presence of the adjuvants alum and CpG oligodeoxynucleotide 1826 provided remarkable immunity, consistently protecting 100% of the A/Sn mice. Immunity was completely reversed by the in vivo depletion of CD8(+) T cells, but not CD4(+) T cells, and was associated with the presence of CD8(+) T cells specific for an epitope located between amino acids 320 and 327 of ASP-2. We concluded that a relatively simple formulation consisting of a recombinant protein with a selected portion of ASP-2, alum, and CpG oligodeoxynucleotide 1826 might be used to cross-prime strong CD8(+)-T-cell-dependent protective immunity against T. cruzi infection.
Figures



Similar articles
-
Protective immunity against trypanosoma cruzi infection in a highly susceptible mouse strain after vaccination with genes encoding the amastigote surface protein-2 and trans-sialidase.Hum Gene Ther. 2004 Sep;15(9):878-86. doi: 10.1089/hum.2004.15.878. Hum Gene Ther. 2004. PMID: 15353042
-
Genetic immunization based on the ubiquitin-fusion degradation pathway against Trypanosoma cruzi.Biochem Biophys Res Commun. 2010 Feb 12;392(3):277-82. doi: 10.1016/j.bbrc.2009.12.166. Epub 2010 Jan 7. Biochem Biophys Res Commun. 2010. PMID: 20059980
-
Efficient protective immunity against Trypanosoma cruzi infection after nasal vaccination with recombinant Sendai virus vector expressing amastigote surface protein-2.Vaccine. 2009 Oct 19;27(44):6154-9. doi: 10.1016/j.vaccine.2009.08.026. Epub 2009 Aug 25. Vaccine. 2009. PMID: 19712768
-
Trypanosoma cruzi infection from the view of CD8+ T cell immunity--an infection model for developing T cell vaccine.Parasitol Int. 2008 Mar;57(1):38-48. doi: 10.1016/j.parint.2007.07.005. Epub 2007 Aug 1. Parasitol Int. 2008. PMID: 17728174 Review.
-
Vaccination approaches against Trypanosoma cruzi infection.Expert Rev Vaccines. 2009 Jul;8(7):921-35. doi: 10.1586/erv.09.45. Expert Rev Vaccines. 2009. PMID: 19538117 Review.
Cited by
-
Oral vaccination with Salmonella enterica as a cruzipain-DNA delivery system confers protective immunity against Trypanosoma cruzi.Infect Immun. 2008 Jan;76(1):324-33. doi: 10.1128/IAI.01163-07. Epub 2007 Oct 29. Infect Immun. 2008. PMID: 17967857 Free PMC article.
-
Epitope Capsid-Incorporation: New Effective Approach for Vaccine Development for Chagas Disease.Pathog Immun. 2016 Fall-Winter;1(2):214-233. doi: 10.20411/pai.v1i2.114. Pathog Immun. 2016. PMID: 27709126 Free PMC article.
-
Biological and immunological characterization of recombinant Yellow Fever 17D viruses expressing a Trypanosoma cruzi Amastigote Surface Protein-2 CD8+ T cell epitope at two distinct regions of the genome.Virol J. 2011 Mar 18;8:127. doi: 10.1186/1743-422X-8-127. Virol J. 2011. PMID: 21418577 Free PMC article.
-
Trans-sialidase from Trypanosoma brucei as a potential target for DNA vaccine development against African trypanosomiasis.Parasitol Res. 2009 Oct;105(5):1223-9. doi: 10.1007/s00436-009-1542-6. Epub 2009 Jul 7. Parasitol Res. 2009. PMID: 19582478
-
Perforin and gamma interferon expression are required for CD4+ and CD8+ T-cell-dependent protective immunity against a human parasite, Trypanosoma cruzi, elicited by heterologous plasmid DNA prime-recombinant adenovirus 5 boost vaccination.Infect Immun. 2009 Oct;77(10):4383-95. doi: 10.1128/IAI.01459-08. Epub 2009 Aug 3. Infect Immun. 2009. PMID: 19651871 Free PMC article.
References
-
- Ackerman, A. L., and P. Cresswell. 2004. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5:678-684. - PubMed
-
- Costa, F., G. Franchin, V. L. Pereira-Chioccola, M. Ribeirão, S. Schenkman, and M. M. Rodrigues. 1998. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine 16:768-774. - PubMed
-
- Fralish, B. H., and R. L. Tarleton. 2003. Genetic immunization with LYT1 or a pool of trans-sialidase genes protects mice from lethal Trypanosoma cruzi infection. Vaccine 21:3070-3080. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

