Brucella abortus virB12 is expressed during infection but is not an essential component of the type IV secretion system
- PMID: 16113325
- PMCID: PMC1231059
- DOI: 10.1128/IAI.73.9.6048-6054.2005
Brucella abortus virB12 is expressed during infection but is not an essential component of the type IV secretion system
Abstract
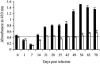
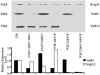
The Brucella abortus virB operon, consisting of 11 genes, virB1 to virB11, and two putative genes, orf12 (virB12) and orf13, encodes a type IV secretion system (T4SS) that is required for intracellular replication and persistent infection in the mouse model. This study was undertaken to determine whether orf12 (virB12) encodes an essential part of the T4SS apparatus. The virB12 gene was found to encode a 17-kDa protein, which was detected in vitro in B. abortus grown to stationary phase. Mice infected with B. abortus 2308 produced an antibody response to the protein encoded by virB12, showing that this gene is expressed during infection. Expression of virB12 was not required for survival in J774 macrophages. VirB12 was also dispensable for the persistence of B. abortus, B. melitensis, and B. suis in mice up to 4 weeks after infection, since deletion mutants lacking virB12 were recovered from splenic tissue at wild-type levels. These results show that VirB12 is not essential for the persistence of the human-pathogenic Brucella spp. in the mouse and macrophage models of infection.
Figures


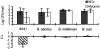

Similar articles
-
The VirB System Plays a Crucial Role in Brucella Intracellular Infection.Int J Mol Sci. 2021 Dec 20;22(24):13637. doi: 10.3390/ijms222413637. Int J Mol Sci. 2021. PMID: 34948430 Free PMC article. Review.
-
VirB12 is a serological marker of Brucella infection in experimental and natural hosts.Clin Vaccine Immunol. 2008 Feb;15(2):208-14. doi: 10.1128/CVI.00374-07. Epub 2007 Dec 12. Clin Vaccine Immunol. 2008. PMID: 18077620 Free PMC article.
-
VirB3 to VirB6 and VirB8 to VirB11, but not VirB7, are essential for mediating persistence of Brucella in the reticuloendothelial system.J Bacteriol. 2008 Jul;190(13):4427-36. doi: 10.1128/JB.00406-08. Epub 2008 May 9. J Bacteriol. 2008. PMID: 18469100 Free PMC article.
-
Differential requirements for VirB1 and VirB2 during Brucella abortus infection.Infect Immun. 2004 Sep;72(9):5143-9. doi: 10.1128/IAI.72.9.5143-5149.2004. Infect Immun. 2004. PMID: 15322008 Free PMC article.
-
Genomic insights into Brucella.Infect Genet Evol. 2021 Jan;87:104635. doi: 10.1016/j.meegid.2020.104635. Epub 2020 Nov 12. Infect Genet Evol. 2021. PMID: 33189905 Review.
Cited by
-
Cytotoxicity in macrophages infected with rough Brucella mutants is type IV secretion system dependent.Infect Immun. 2008 Jan;76(1):30-7. doi: 10.1128/IAI.00379-07. Epub 2007 Oct 15. Infect Immun. 2008. PMID: 17938217 Free PMC article.
-
The VirB System Plays a Crucial Role in Brucella Intracellular Infection.Int J Mol Sci. 2021 Dec 20;22(24):13637. doi: 10.3390/ijms222413637. Int J Mol Sci. 2021. PMID: 34948430 Free PMC article. Review.
-
Different Impacts of MucR Binding to the babR and virB Promoters on Gene Expression in Brucella abortus 2308.Biomolecules. 2020 May 19;10(5):788. doi: 10.3390/biom10050788. Biomolecules. 2020. PMID: 32438765 Free PMC article.
-
Bacterial Type IV secretion systems: versatile virulence machines.Future Microbiol. 2012 Feb;7(2):241-57. doi: 10.2217/fmb.11.150. Future Microbiol. 2012. PMID: 22324993 Free PMC article. Review.
-
VirB12 is a serological marker of Brucella infection in experimental and natural hosts.Clin Vaccine Immunol. 2008 Feb;15(2):208-14. doi: 10.1128/CVI.00374-07. Epub 2007 Dec 12. Clin Vaccine Immunol. 2008. PMID: 18077620 Free PMC article.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
-
- Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. - PMC - PubMed
-
- Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3:159-168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

