Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice
- PMID: 16113331
- PMCID: PMC1231090
- DOI: 10.1128/IAI.73.9.6101-6109.2005
Therapeutic efficacy of high-dose intravenous immunoglobulin in Mycobacterium tuberculosis infection in mice
Abstract
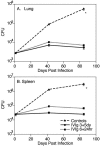
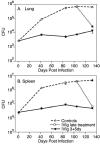
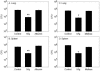
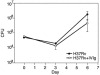
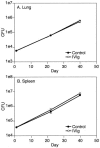
Intravenous immunoglobulin (IVIg) is used to treat patients with primary antibody deficiencies and, at high doses, to treat a range of autoimmune and inflammatory disorders. With high-dose IVIg (hdIVIg), immunomodulatory mechanisms act on a range of cells, including T cells, B cells, and dendritic cells. Here, we demonstrate that the treatment of M. tuberculosis-infected mice with a single cycle of hdIVIg resulted in substantially reduced bacterial loads in the spleen and lungs when administered at either an early or late stage of infection. Titration of the IVIg showed a clear dose-response effect. There was no reduction in bacterial load when mice were given equimolar doses of another human protein, human serum albumin, or maltose, the stabilizing agent in the IVIg preparation. HdIVIg in vitro had no inhibitory effect on the growth of M. tuberculosis in murine bone marrow-derived macrophages. In addition, the effect of hdIVIg on bacterial loads was not observed in nude mice, suggesting the involvement of conventional T cells. Analysis of T cells infiltrating the lungs revealed only small increases in CD8(+) but not CD4(+) T-cell numbers in hdIVIg-treated mice. The mechanism of action of hdIVIg against tuberculosis in mice remains to be determined. Nevertheless, since hdIVIg is already widely used clinically, the magnitude and long duration of the therapeutic effect seen here suggest that IVIg, or components of it, may find ready application as an adjunct to therapy of human tuberculosis.
Figures


References
-
- Akiyama, K., S. Ebihara, A. Yada, K. Matsumura, S. Aiba, T. Nukiwa, and T. Takai. 2003. Targeting apoptotic tumor cells to Fc gamma R provides efficient and versatile vaccination against tumors by dendritic cells. J. Immunol. 170:1641-1648. - PubMed
-
- Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. - PubMed
-
- Bayry, J., S. Lacroix-Desmazes, V. Donkova-Petrini, C. Carbonneil, N. Misra, Y. Lepelletier, S. Delignat, S. Varambally, E. Oksenhendler, Y. Levy, M. Debre, M. D. Kazatchkine, O. Hermine, and S. V. Kaveri. 2004. Natural antibodies sustain differentiation and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 101:14210-14215. - PMC - PubMed
-
- Belz, G. T., F. R. Carbone, and W. R. Heath. 2002. Cross-presentation of antigens by dendritic cells. Crit. Rev. Immunol. 22:439-448. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

