Characterization of FimH adhesins expressed by Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum: reconstitution of mannose-binding properties by single amino acid substitution
- PMID: 16113346
- PMCID: PMC1231085
- DOI: 10.1128/IAI.73.9.6187-6190.2005
Characterization of FimH adhesins expressed by Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum: reconstitution of mannose-binding properties by single amino acid substitution
Abstract
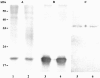
Recombinant FimH adhesins of type 1 fimbriae from Salmonella enterica serovar Gallinarum biovars Gallinarum and Pullorum, in contrast to those of Salmonella enterica serovar Typhimurium, did not bind to high-mannose oligosaccharides or to human colon carcinoma HT-29 cells. However, mutated FimH proteins from biovar Gallinarum and biovar Pullorum, in which the isoleucine at position 78 was replaced by the threonine found in S. enterica serovar Typhimurium, bound well to glycoproteins carrying high-mannose oligosaccharides and colon carcinoma cells. The loss of sugar-binding properties by biovar Gallinarum and biovar Pullorum FimH adhesins, which are a part of the type 1 fimbriae, is most probably the result of a single T78I mutation, as was proven by site-directed mutagenesis of FimH proteins.
Figures



Similar articles
-
Differentiation of Salmonella Gallinarum biovar Gallinarum from Salmonella Gallinarum biovar Pullorum by PCR-RFLP of the fimH gene.J Vet Med B Infect Dis Vet Public Health. 2005 Jun;52(5):214-8. doi: 10.1111/j.1439-0450.2005.00846.x. J Vet Med B Infect Dis Vet Public Health. 2005. PMID: 16115094
-
FimH adhesin from host unrestricted Salmonella Enteritidis binds to different glycoprotein ligands expressed by enterocytes from sheep, pig and cattle than FimH adhesins from host restricted Salmonella Abortus-ovis, Salmonella Choleraesuis and Salmonella Dublin.Vet Microbiol. 2013 Oct 25;166(3-4):550-7. doi: 10.1016/j.vetmic.2013.07.004. Epub 2013 Jul 17. Vet Microbiol. 2013. PMID: 23910950
-
Decreased colonization of chicks by Salmonella enterica serovar Gallinarum expressing mannose-sensitive FimH adhesin from Salmonella enterica serovar Enteritidis.Vet Microbiol. 2012 Jul 6;158(1-2):205-10. doi: 10.1016/j.vetmic.2012.01.029. Epub 2012 Feb 3. Vet Microbiol. 2012. PMID: 22364838
-
Salmonella enterica Serovar Gallinarum Biovars Pullorum and Gallinarum in Poultry: Review of Pathogenesis, Antibiotic Resistance, Diagnosis and Control in the Genomic Era.Antibiotics (Basel). 2023 Dec 25;13(1):23. doi: 10.3390/antibiotics13010023. Antibiotics (Basel). 2023. PMID: 38247582 Free PMC article. Review.
-
The immunobiology of avian systemic salmonellosis.Vet Immunol Immunopathol. 2009 Mar 15;128(1-3):53-9. doi: 10.1016/j.vetimm.2008.10.295. Epub 2008 Oct 17. Vet Immunol Immunopathol. 2009. PMID: 19070366 Review.
Cited by
-
The Typhi colonization factor (Tcf) is encoded by multiple non-typhoidal Salmonella serovars but exhibits a varying expression profile and interchanging contribution to intestinal colonization.Virulence. 2017 Nov 17;8(8):1791-1807. doi: 10.1080/21505594.2017.1380766. Epub 2017 Nov 10. Virulence. 2017. PMID: 28922626 Free PMC article.
-
The plasmid-encoded Ipf and Klf fimbriae display different expression and varying roles in the virulence of Salmonella enterica serovar Infantis in mouse vs. avian hosts.PLoS Pathog. 2017 Aug 17;13(8):e1006559. doi: 10.1371/journal.ppat.1006559. eCollection 2017 Aug. PLoS Pathog. 2017. PMID: 28817673 Free PMC article.
-
Diversification of the Salmonella fimbriae: a model of macro- and microevolution.PLoS One. 2012;7(6):e38596. doi: 10.1371/journal.pone.0038596. Epub 2012 Jun 12. PLoS One. 2012. PMID: 22701679 Free PMC article.
-
The Possible Influence of Non-synonymous Point Mutations within the FimA Adhesin of Non-typhoidal Salmonella (NTS) Isolates in the Process of Host Adaptation.Front Microbiol. 2017 Oct 17;8:2030. doi: 10.3389/fmicb.2017.02030. eCollection 2017. Front Microbiol. 2017. PMID: 29089942 Free PMC article.
-
Glycan-mediated adhesion mechanisms in antibiotic-resistant bacteria.BBA Adv. 2025 Mar 14;7:100156. doi: 10.1016/j.bbadva.2025.100156. eCollection 2025. BBA Adv. 2025. PMID: 40207210 Free PMC article.
References
-
- Boratyński, J., and R. Roy. 1998. High temperature conjugation of proteins with carbohydrates. Glycoconj. J. 15:131-138. - PubMed
-
- Crichton, P. B., D. E. Yakubu, D. C. Old, and S. Clegg. 1989. Immunological and genetical relatedness of type-1 and type-2 fimbriae in salmonellas of serotypes Gallinarum, Pullorum and Typhimurium. J. Appl. Bacteriol. 67:283-291. - PubMed
-
- Doran, J. L., S. K. Collinson, C. M. Kay, P. A. Banser, J. Burian, C. K. Munro, S. H. Lee, J. M. Somers, E. C. Todd, and W. W. Kay. 1994. fimA and tctC based DNA diagnostics for Salmonella. Mol. Cell. Probes 8:291-310. - PubMed
-
- Duguid, J. P., E. S. Anderson, and I. Campbell. 1966. Fimbriae and adhesive properties in Salmonellae. J. Pathol. Bacteriol. 92:107-138. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

