Influence of format on in vitro penetration of antibody fragments through porcine cornea
- PMID: 16113383
- PMCID: PMC1772801
- DOI: 10.1136/bjo.2005.066225
Influence of format on in vitro penetration of antibody fragments through porcine cornea
Abstract
Aim: Antibody fragments, appropriately formulated, can penetrate through the ocular surface and thus have potential as therapeutic agents. The aim was to investigate the influence of protein fragment format on the kinetics and extent of ocular penetration in vitro.
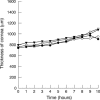
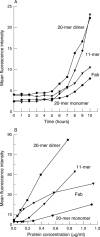
Methods: Immunoglobulin single chain variable domain fragments of a murine monoclonal antibody with specificity for rat CD4 were engineered with a 20 or 11 amino acid linker by assembly polymerase chain reaction, expressed in Escherichia coli and purified by chromatography. Fab fragments of the parental antibody were prepared by papain digestion. Antibody fragments were formulated with a penetration and a viscosity enhancer and were applied to the surface of perfused pig corneas for up to 10 hours in vitro. Penetration was quantified by flow cytometry on rat thymocytes.
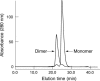
Results: 20-mer antibody fragments formed natural monomers and dimers following purification that could be separately isolated, while 11-mer fragments were dimeric. All formats of fragment (20-mer monomers and dimers, 11-mer dimers, Fab) showed penetration through the pig cornea after 6 hours of intermittent topical administration.
Conclusion: Antibody fragments of different shapes and sizes can penetrate the cornea after topical administration, thereby increasing the potential of this class of proteins for topical ophthalmic use.
Figures


Similar articles
-
Topically applied antibody fragments penetrate into the back of the rabbit eye.Eye (Lond). 2005 Aug;19(8):910-3. doi: 10.1038/sj.eye.6701669. Eye (Lond). 2005. PMID: 15359243
-
Penetration of engineered antibody fragments into the eye.Clin Exp Immunol. 2002 Apr;128(1):67-74. doi: 10.1046/j.1365-2249.2002.01808.x. Clin Exp Immunol. 2002. PMID: 11982592 Free PMC article.
-
Engineered antibody intervention strategies for Alzheimer's disease and related dementias by targeting amyloid and toxic oligomers.Protein Eng Des Sel. 2009 Mar;22(3):199-208. doi: 10.1093/protein/gzn052. Epub 2008 Oct 16. Protein Eng Des Sel. 2009. PMID: 18927231
-
Helix-stabilized Fv (hsFv) antibody fragments: substituting the constant domains of a Fab fragment for a heterodimeric coiled-coil domain.J Mol Biol. 2001 Sep 7;312(1):221-8. doi: 10.1006/jmbi.2001.4915. J Mol Biol. 2001. PMID: 11545598
-
Pharmacokinetics and biodistribution of genetically-engineered antibodies.Q J Nucl Med. 1998 Dec;42(4):225-41. Q J Nucl Med. 1998. PMID: 9973838 Review.
Cited by
-
Safety and tolerability of pooled human immune globulins after topical ophthalmic administration in New Zealand White rabbits.Cutan Ocul Toxicol. 2024 Sep;43(3):227-231. doi: 10.1080/15569527.2024.2381207. Epub 2024 Jul 31. Cutan Ocul Toxicol. 2024. PMID: 39086095 Free PMC article.
-
3-hydroxykynurenine suppresses CD4+ T-cell proliferation, induces T-regulatory-cell development, and prolongs corneal allograft survival.Invest Ophthalmol Vis Sci. 2011 Apr 22;52(5):2640-8. doi: 10.1167/iovs.10-5793. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21212175 Free PMC article.
-
Successful regression of newly formed corneal neovascularization by subconjunctival injection of bevacizumab in patients with chemical burns.Front Med (Lausanne). 2023 Jun 22;10:1210765. doi: 10.3389/fmed.2023.1210765. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37425330 Free PMC article.
-
Topical Bevacizumab for the Treatment of Ocular Surface Squamous Neoplasia.J Ocul Pharmacol Ther. 2015 Oct;31(8):487-90. doi: 10.1089/jop.2014.0158. Epub 2015 Jun 26. J Ocul Pharmacol Ther. 2015. PMID: 26114378 Free PMC article. Clinical Trial.
-
Topical Anti-TNFα Agent Licaminlimab (OCS-02) Relieves Persistent Ocular Discomfort in Severe Dry Eye Disease: A Randomized Phase II Study.Clin Ophthalmol. 2022 Jul 6;16:2167-2177. doi: 10.2147/OPTH.S366836. eCollection 2022. Clin Ophthalmol. 2022. PMID: 35821785 Free PMC article. Clinical Trial.
References
-
- Prausnitz MR, Noonan JS. Permeability of cornea, sclera, and conjunctiva: a literature analysis for drug delivery to the eye. J Pharm Sci 1998;87:1479–88. - PubMed
-
- Tonjum AM. Permeability of horseradish peroxidase in the rabbit corneal epithelium. Acta Ophthalmol 1974;52:650–8. - PubMed
-
- Grass GM, Robinson JR. Mechanisms of corneal drug penetration. II: Ultrastructural analysis of potential pathways for drug movement, J Pharm Sci 1988;77:15–23. - PubMed
-
- Hamalainen KM, Kananen K, Auriola S, et al. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci 1997;38:627–34. - PubMed
-
- Lee VH, Carson LW, Takemoto KA. Macromolecular drug absorption in the albino rabbit eye. Int J Pharm 1986;29:43–51.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
