New generation live vaccines against human respiratory syncytial virus designed by reverse genetics
- PMID: 16113487
- PMCID: PMC2713317
- DOI: 10.1513/pats.200501-011AW
New generation live vaccines against human respiratory syncytial virus designed by reverse genetics
Abstract
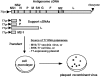
Development of a live pediatric vaccine against human respiratory syncytial virus (RSV) is complicated by the need to immunize young infants and the difficulty in balancing attenuation and immunogenicity. The ability to introduce desired mutations into infectious virus by reverse genetics provides a method for identifying and designing highly defined attenuating mutations. These can be introduced in combinations as desired to achieve gradations of attenuation. Attenuation is based on several strategies: multiple independent temperature-sensitive point mutations in the polymerase, a temperature-sensitive point mutation in a transcription signal, a set of non-temperature-sensitive mutations involving several genes, deletion of a viral RNA synthesis regulatory protein, and deletion of viral IFN alpha/beta antagonists. The genetic stability of the live vaccine can be increased by judicious choice of mutations. The virus also can be engineered to increase the level of expression of the protective antigens. Protective antigens from antigenically distinct RSV strains can be added or swapped to increase the breadth of coverage. Alternatively, the major RSV protective antigens can be expressed from transcription units added to an attenuated parainfluenza vaccine virus, making a bivalent vaccine. This would obviate the difficulties inherent in the fragility and inefficient in vitro growth of RSV, simplifying vaccine design and use.
Figures


References
-
- Collins PL, Hill MG, Camargo E, Grosfeld H, Chanock RM, Murphy BR. Production of infectious human respiratory syncytial virus from cloned cDNA confirms an essential role for the transcription elongation factor from the 5′ proximal open reading frame of the M2 mRNA in gene expression and provides a capability for vaccine development. Proc Natl Acad Sci USA 1995;92:11563–11567. - PMC - PubMed
-
- Conzelmann KK. Reverse genetics of mononegavirales. Curr Top Microbiol Immunol 2004;283:1–41. - PubMed
-
- Collins PL, Chanock RM, Murphy BR. Respiratory syncytial virus. In: Knipe DM, Howley, PM, editors. Fields Virology, 4th ed. Philadelphia, PA: Lippincott, Williams and Wilkins; 2001. p. 1443–1485.
-
- McCarthy CA, Hall CB. Respiratory syncytial virus: concerns and control. Pediatr Rev 2003;24:301–309. - PubMed
-
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. JAMA 1999;282:1440–1446. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
