Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization
- PMID: 16113676
- PMCID: PMC1351395
- DOI: 10.1038/ncb1293
Phospho-caveolin-1 mediates integrin-regulated membrane domain internalization
Abstract
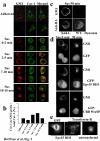
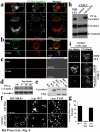
Growth of normal cells is anchorage dependent because signalling through multiple pathways including Erk, phosphatidylinositol-3-OH kinase (PI(3)K) and Rac requires integrin-mediated cell adhesion. Components of these pathways localize to low-density, cholesterol-rich domains in the plasma membrane named 'lipid rafts' or 'cholesterol-enriched membrane microdomains' (CEMM). We previously reported that integrin-mediated adhesion regulates CEMM transport such that cell detachment from the extracellular matrix triggers CEMM internalization and clearance from the plasma membrane. We now report that this internalization is mediated by dynamin-2 and caveolin-1. Internalization requires phosphorylation of caveolin-1 on Tyr 14. A shift in localization of phospho-caveolin-1 from focal adhesions to caveolae induces CEMM internalization upon cell detachment, which mediates inhibition of Erk, PI(3)K and Rac. These data define a novel molecular mechanism for growth and tumour suppression by caveolin-1.
Figures


References
-
- Edidin M. The state of lipid rafts: from model membranes to cells. Annu Rev Biophys Biomol Struct. 2003;32:257–83. - PubMed
-
- Lagerholm BC, Weinreb GE, Jacobson K, Thompson NL. Detecting Microdomains in Intact Cell Membranes. Annu Rev Phys Chem. 2005;56:309–336. - PubMed
-
- Grande A, Echarri A, del Pozo MA. Integrin regulation of membrane domain trafficking and Rac targeting. Biochemical Society Transactions. 2005;33:609–613. - PubMed
-
- del Pozo MA, et al. Integrins regulate Rac targeting by internalization of membrane domains. Science. 2004;303:839–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

