PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival
- PMID: 16113679
- PMCID: PMC2771163
- DOI: 10.1038/ncb1288
PDGFRbeta+ perivascular progenitor cells in tumours regulate pericyte differentiation and vascular survival
Abstract
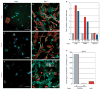
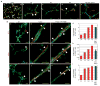
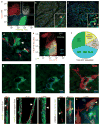
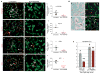
The microvasculature consists of endothelial cells and their surrounding pericytes. Few studies on the regulatory mechanisms of tumour angiogenesis have focused on pericytes. Here we report the identification of tumour-derived PDGFRbeta (+) (platelet-derived growth factor receptor beta) progenitor perivascular cells (PPCs) that have the ability to differentiate into pericytes and regulate vessel stability and vascular survival in tumours. A subset of PDGFRbeta (+) PPCs is recruited from bone marrow to perivascular sites in tumours. Specific inhibition of PDGFRbeta signalling eliminates PDGFRbeta (+) PPCs and mature pericytes around tumour vessels, leading to vascular hyperdilation and endothelial cell apoptosis in pancreatic islet tumours of transgenic Rip1Tag2 mice.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

References
-
- Sims DE. Diversity within pericytes. Clin Exp Pharmacol Physiol. 2000;27:842–846. - PubMed
-
- Gerhardt H, Betsholtz C. Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res. 2003;314:15–23. - PubMed
-
- Cleaver O, Melton DA. Endothelial signaling during development. Nature Med. 2003;9:661–668. - PubMed
-
- Hirschi KK, D’Amore PA. Pericytes in the microvasculature. Cardiovasc Res. 1996;32:687–698. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature Rev Cancer. 2003;3:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

