Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis
- PMID: 16113800
- PMCID: PMC2893037
- DOI: 10.1160/TH04-09-0566
Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis
Abstract
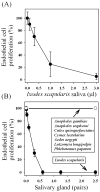
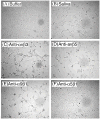
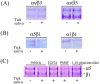
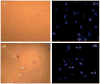
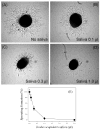
We report for the first time that saliva of the hard tick and Lyme disease vector, Ixodes scapularis, is a potent inhibitor of angiogenesis. Saliva (< or = 1:500 dilutions) or salivary gland (0.1-0.5 pairs/assay) dose-dependently inhibits microvascular endothelial cell (MVEC) proliferation. Inhibition was also detected with the saliva of the cattle tick Boophilus microplus but not with the salivary gland of Anopheles gambiae, An. stephensi, Lutzomyia longipalpis, Phlebotomus papatasi, Aedes aegypti, Culex quinquefasciatus, and Cimex lectularius. Inhibition of MVEC proliferation by I. Scapularis saliva was accompanied by a change in cell shape (shrinkage of the cytoplasm with loss of cell-cell interactions) and apoptosis which was estimated by expression of phosphatidylserine using the Apopercentage dye, and by a typical pattern of chromatin margination, condensation, and fragmentation as revealed by nuclear staining with Hoechst 33258. The effect of saliva appears to be mediated by endothelial cell alpha5beta1 integrin, because monoclonal antibodies against this but not alphavbeta3, alphavbeta5, alpha9beta1, or alpha2beta1 integrins remarkably block its effect. In addition, SDS/PAGE shows that saliva specifically degrades purified alpha5beta1 but not alphavbeta5 or alphavbeta3 integrins. Incubation of saliva with EDTA and 1,10-phenanthroline, but not phenylmethylsulfonyl fluoride (PMSF), inhibits saliva-dependent degradation of purified alpha5beta1 integrin, suggesting that a metalloprotease is responsible for the activity. Finally, saliva at < or = 1:1,000 dilutions blocks sprouting formation from chick embryo aorta implanted in Matrigel, an in vitro model of angiogenesis. These findings introduce the concept that tick saliva is a negative modulator of angiogenesis-dependent wound healing and tissue repair, therefore allowing ticks to feed for days. Inhibition of angiogenesis was hitherto an unidentified biologic property of the saliva of any blood-sucking arthropod studied so far. Its presence in tick saliva may be regarded as an additional source of angiogenesis inhibitors with potential applications for the study of both vector and vascular biology.
Figures

Similar articles
-
Purification of a serine protease and evidence for a protein C activator from the saliva of the tick, Ixodes scapularis.Toxicon. 2014 Jan;77:32-9. doi: 10.1016/j.toxicon.2013.10.025. Epub 2013 Oct 31. Toxicon. 2014. PMID: 24184517 Free PMC article.
-
Tick-Host Range Adaptation: Changes in Protein Profiles in Unfed Adult Ixodes scapularis and Amblyomma americanum Saliva Stimulated to Feed on Different Hosts.Front Cell Infect Microbiol. 2017 Dec 19;7:517. doi: 10.3389/fcimb.2017.00517. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29312895 Free PMC article.
-
Identification of an IL-2 binding protein in the saliva of the Lyme disease vector tick, Ixodes scapularis.J Immunol. 2001 Apr 1;166(7):4319-26. doi: 10.4049/jimmunol.166.7.4319. J Immunol. 2001. PMID: 11254684
-
Regulation of endothelial cell integrin function and angiogenesis by COX-2, cAMP and Protein Kinase A.Thromb Haemost. 2003 Oct;90(4):577-85. doi: 10.1160/TH03-03-0196. Thromb Haemost. 2003. PMID: 14515176 Review.
-
Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks.Ticks Tick Borne Dis. 2018 Mar;9(3):535-542. doi: 10.1016/j.ttbdis.2018.01.002. Epub 2018 Jan 31. Ticks Tick Borne Dis. 2018. PMID: 29398603 Free PMC article. Review.
Cited by
-
Interactions between Borrelia burgdorferi and ticks.Nat Rev Microbiol. 2020 Oct;18(10):587-600. doi: 10.1038/s41579-020-0400-5. Epub 2020 Jul 10. Nat Rev Microbiol. 2020. PMID: 32651470 Free PMC article. Review.
-
Bioinformatic Analysis of Ixodes ricinus Long Non-Coding RNAs Predicts Their Binding Ability of Host miRNAs.Int J Mol Sci. 2022 Aug 28;23(17):9761. doi: 10.3390/ijms23179761. Int J Mol Sci. 2022. PMID: 36077158 Free PMC article.
-
The sialotranscriptome of Amblyomma triste, Amblyomma parvum and Amblyomma cajennense ticks, uncovered by 454-based RNA-seq.Parasit Vectors. 2014 Sep 8;7:430. doi: 10.1186/1756-3305-7-430. Parasit Vectors. 2014. PMID: 25201527 Free PMC article.
-
Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: a comparative survey and review.J Ethnobiol Ethnomed. 2017 Feb 7;13(1):9. doi: 10.1186/s13002-017-0136-0. J Ethnobiol Ethnomed. 2017. PMID: 28173820 Free PMC article. Review.
-
Serine Protease Inhibitors in Ticks: An Overview of Their Role in Tick Biology and Tick-Borne Pathogen Transmission.Front Cell Infect Microbiol. 2017 May 22;7:199. doi: 10.3389/fcimb.2017.00199. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28589099 Free PMC article. Review.
References
-
- Folkman J, Shing T. Angiogenesis. J Biol Chem. 1992;167:10931–10934. - PubMed
-
- Risau W. Mechanism of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- Li J, Zhang YP, Kirsner RS. Angiogenesis and wound repair: angiogenic growth factors and the extracellular matrix. Microscopy Research and Technique. 2003;60:107–114. - PubMed
-
- Neal MS. Angiogenesis: is it the key to controlling the healing process? J Wound Care. 2001;10:281–287. - PubMed
-
- Ribeiro JM, Francischetti IM. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol. 2003;48:73–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

