Replicative homeostasis II: influence of polymerase fidelity on RNA virus quasispecies biology: implications for immune recognition, viral autoimmunity and other "virus receptor" diseases
- PMID: 16115320
- PMCID: PMC1260030
- DOI: 10.1186/1743-422X-2-70
Replicative homeostasis II: influence of polymerase fidelity on RNA virus quasispecies biology: implications for immune recognition, viral autoimmunity and other "virus receptor" diseases
Abstract
Much of the worlds' population is in active or imminent danger from established infectious pathogens, while sporadic and pandemic infections by these and emerging agents threaten everyone. RNA polymerases (RNApol) generate enormous genetic and consequent antigenic heterogeneity permitting both viruses and cellular pathogens to evade host defences. Thus, RNApol causes more morbidity and premature mortality than any other molecule. The extraordinary genetic heterogeneity defining viral quasispecies results from RNApol infidelity causing rapid cumulative genomic RNA mutation a process that, if uncontrolled, would cause catastrophic loss of sequence integrity and inexorable quasispecies extinction. Selective replication and replicative homeostasis, an epicyclical regulatory mechanism dynamically linking RNApol fidelity and processivity with quasispecies phenotypic diversity, modulating polymerase fidelity and, hence, controlling quasispecies behaviour, prevents this happening and also mediates immune escape. Perhaps more importantly, ineluctable generation of broad phenotypic diversity after viral RNA is translated to protein quasispecies suggests a mechanism of disease that specifically targets, and functionally disrupts, the host cell surface molecules--including hormone, lipid, cell signalling or neurotransmitter receptors--that viruses co-opt for cell entry. This mechanism--"Viral Receptor Disease (VRD)"--may explain so-called "viral autoimmunity", some classical autoimmune disorders and other diseases, including type II diabetes mellitus, and some forms of obesity. Viral receptor disease is a unifying hypothesis that may also explain some diseases with well-established, but multi-factorial and apparently unrelated aetiologies--like coronary artery and other vascular diseases--in addition to diseases like schizophrenia that are poorly understood and lack plausible, coherent, pathogenic explanations.
Figures
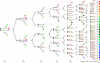
 ) are introduced into each RNA template synthesized, and progressively accumulate, resulting in an RNA quasispecies with sequence progressively divergent from consensus sequence. Translation results in a spectrum of proteins (
) are introduced into each RNA template synthesized, and progressively accumulate, resulting in an RNA quasispecies with sequence progressively divergent from consensus sequence. Translation results in a spectrum of proteins ( ,
,  ,
,  , etc.) with properties that also vary progressively from wild-type sequence (
, etc.) with properties that also vary progressively from wild-type sequence ( ) to highly variant proteins (
) to highly variant proteins ( ,
,  , etc.). Some RNAs will be so abnormal that translation or replication fails or is truncated (
, etc.). Some RNAs will be so abnormal that translation or replication fails or is truncated ( ), while others will code for grossly defective proteins (
), while others will code for grossly defective proteins ( ,
,  etc.).
etc.).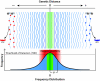
 ,
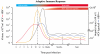
,  ) accumulate and RNA sequence progressively diverges from consensus sequence (0) the probability of that RNA sequence and corresponding protein (e.g. envelope, Env.) arising falls rapidly. Standard deviation (σ) of frequency distribution is proportional to RNApol fidelity.
) accumulate and RNA sequence progressively diverges from consensus sequence (0) the probability of that RNA sequence and corresponding protein (e.g. envelope, Env.) arising falls rapidly. Standard deviation (σ) of frequency distribution is proportional to RNApol fidelity.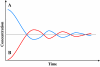
 ) or B (
) or B ( ) and product:enzyme interactions occur such that A:E produce B while B:E favour A, then high initial concentrations of A (or B) will cause rapid synthesis of B (or A). Equilibrium ultimately develops irrespective of starting conditions.
) and product:enzyme interactions occur such that A:E produce B while B:E favour A, then high initial concentrations of A (or B) will cause rapid synthesis of B (or A). Equilibrium ultimately develops irrespective of starting conditions.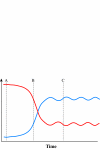
 ) and fidelity (
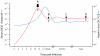
) and fidelity ( ) predicted by replicative homeostasis. Initial state (A, corresponding to panel A, Figure 7): in a newly infected cell, high-affinity wild-type:RNApol interactions will predominate resulting in high RNApol processivity but low fidelity causing high-level viraemia with broad virus phenotypic spectrum, maximizing cell tropism. Intracellular accumulation of variant viral proteins (B, c.f. panel B, Figure 7) reduces RNApol processivity but increases fidelity reducing viral RNA synthesis and consequently, viraemia before a dynamic, fluctuating equilibrium (C, c.f. panel C or D, Figure 7) develops in which inhibition of RNApol by variant viral proteins is balanced by increases in RNApol fidelity (with consequent synthesis of wild-type viral products tending to cause high RNApol processivity).
) predicted by replicative homeostasis. Initial state (A, corresponding to panel A, Figure 7): in a newly infected cell, high-affinity wild-type:RNApol interactions will predominate resulting in high RNApol processivity but low fidelity causing high-level viraemia with broad virus phenotypic spectrum, maximizing cell tropism. Intracellular accumulation of variant viral proteins (B, c.f. panel B, Figure 7) reduces RNApol processivity but increases fidelity reducing viral RNA synthesis and consequently, viraemia before a dynamic, fluctuating equilibrium (C, c.f. panel C or D, Figure 7) develops in which inhibition of RNApol by variant viral proteins is balanced by increases in RNApol fidelity (with consequent synthesis of wild-type viral products tending to cause high RNApol processivity).
Similar articles
-
Replicative homeostasis: a fundamental mechanism mediating selective viral replication and escape mutation.Virol J. 2005 Feb 11;2:10. doi: 10.1186/1743-422X-2-10. Virol J. 2005. PMID: 15707489 Free PMC article.
-
RNA virus quasispecies: significance for viral disease and epidemiology.Infect Agents Dis. 1994 Aug;3(4):201-14. Infect Agents Dis. 1994. PMID: 7827789 Review.
-
Replicative homeostasis: a mechanism of viral persistence.Med Hypotheses. 2004;63(3):515-23. doi: 10.1016/j.mehy.2004.02.050. Med Hypotheses. 2004. PMID: 15288380
-
RNA Viruses and RNAi: Quasispecies Implications for Viral Escape.Viruses. 2015 Jun 19;7(6):3226-40. doi: 10.3390/v7062768. Viruses. 2015. PMID: 26102581 Free PMC article. Review.
-
The viral manipulation of the host cellular and immune environments to enhance propagation and survival: a focus on RNA viruses.J Leukoc Biol. 2002 Sep;72(3):429-39. J Leukoc Biol. 2002. PMID: 12223509 Review.
Cited by
-
Correlation between pre-treatment quasispecies complexity and treatment outcome in chronic HCV genotype 3a.Virol J. 2008 Jul 9;5:78. doi: 10.1186/1743-422X-5-78. Virol J. 2008. PMID: 18613968 Free PMC article.
-
Persistent host markers in pandemic and H5N1 influenza viruses.J Virol. 2007 Oct;81(19):10292-9. doi: 10.1128/JVI.00921-07. Epub 2007 Jul 25. J Virol. 2007. PMID: 17652405 Free PMC article.
-
Replicative homeostasis III: implications for antiviral therapy and mechanisms of response and non-response.Virol J. 2007 Mar 13;4:29. doi: 10.1186/1743-422X-4-29. Virol J. 2007. PMID: 17355620 Free PMC article.
-
Tyr82 Amino Acid Mutation in PB1 Polymerase Induces an Influenza Virus Mutator Phenotype.J Virol. 2019 Oct 29;93(22):e00834-19. doi: 10.1128/JVI.00834-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462570 Free PMC article.
-
Estimating the Time to Diagnosis and the Chance of Spontaneous Clearance During Acute Hepatitis C in Human Immunodeficiency Virus-Infected Individuals.Open Forum Infect Dis. 2017 Jan 31;4(1):ofw235. doi: 10.1093/ofid/ofw235. eCollection 2017 Winter. Open Forum Infect Dis. 2017. PMID: 28480234 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

