Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT)
- PMID: 16115871
- PMCID: PMC1352312
- DOI: 10.1074/jbc.M507924200
Retinoid absorption and storage is impaired in mice lacking lecithin:retinol acyltransferase (LRAT)
Abstract
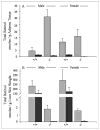
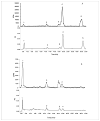
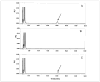
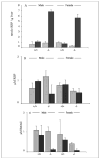
Lecithin:retinol acyltransferase (LRAT) is believed to be the predominant if not the sole enzyme in the body responsible for the physiologic esterification of retinol. We have studied Lrat-deficient (Lrat-/-) mice to gain a better understanding of how these mice take up and store dietary retinoids and to determine whether other enzymes may be responsible for retinol esterification in the body. Although the Lrat-/- mice possess only trace amounts of retinyl esters in liver, lung, and kidney, they possess elevated (by 2-3-fold) concentrations of retinyl esters in adipose tissue compared with wild type mice. These adipose retinyl ester depots are mobilized in times of dietary retinoid insufficiency. We further observed an up-regulation (3-4-fold) in the level of cytosolic retinol-binding protein type III (CRBPIII) in adipose tissue of Lrat-/- mice. Examination by electron microscopy reveals a striking total absence of large lipid-containing droplets that normally store hepatic retinoid within the hepatic stellate cells of Lrat-/- mice. Despite the absence of significant retinyl ester stores and stellate cell lipid droplets, the livers of Lrat-/- mice upon histologic analysis appear normal and show no histological signs of liver fibrosis. Lrat-/- mice absorb dietary retinol primarily as free retinol in chylomicrons; however, retinyl esters are also present within the chylomicron fraction obtained from Lrat-/- mice. The fatty acyl composition of these (chylomicron) retinyl esters suggests that they are synthesized via an acyl-CoA-dependent process suggesting the existence of a physiologically significant acyl-CoA:retinol acyltransferase.
Figures


References
-
- Moore, T. (1957) Vitamin A, Elsevier Publishing Co., Amsterdam
-
- Wald G. Nature. 1968;219:800–807. - PubMed
-
- McBee JK, Palczweski K, Baehr W, Pepperberg DR. Prog Retin Eye Res. 2001;20:469–529. - PubMed
-
- Gudas, L. J., Sporn, M. B., and Roberts, A. B. (1994) in The Retinoids, Biology, Chemistry and Medicine (Sporn, M. B., Roberts, A. B., and Goodman, D. S., eds) 2nd Ed., pp. 443–520, Raven Press, Ltd., New York
-
- Balmer JE, Blomhoff R. J Lipid Res. 2002;43:1773–1808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

