Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis
- PMID: 16116191
- PMCID: PMC3110086
- DOI: 10.4049/jimmunol.175.5.3033
Conditional Fas-associated death domain protein (FADD): GFP knockout mice reveal FADD is dispensable in thymic development but essential in peripheral T cell homeostasis
Abstract
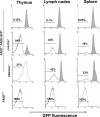
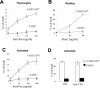
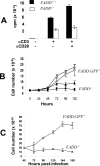
Fas-associated death domain protein (FADD)/mediator of receptor-induced toxicity-1 is required for signaling induced by death receptors such as Fas. In earlier studies, FADD-deficient mice died in utero, and a FADD deficiency in embryonic stem cells inhibited T cell production in viable FADD-/- -->RAG-1-/- chimeras. To analyze the temporal requirement of FADD in the development and function in the T lineage, it is necessary to establish viable mutant mice producing detectable FADD-deficient T cells. We generated mice that express a functional FADD:GFP fusion gene reconstituting normal embryogenesis and lymphopoiesis in the absence of the endogenous FADD. Efficient T cell-specific deletion of FADD:GFP was achieved, as indicated by the presence of a high percentage of GFP-negative thymocytes and peripheral T cells in mice expressing Lck-Cre or CD4-Cre. Sorted GFP-negative thymocytes and peripheral T cells contained undetectable levels of FADD and were resistant to apoptosis induced by Fas, TNF, and TCR restimulation. These T cell-specific FADD-deficient mice contain normal thymocyte numbers, but fewer peripheral T cells. Purified peripheral FADD-deficient T cells failed to undergo extensive homeostatic expansion after adoptive transfer into lymphocyte-deficient hosts, and responded poorly to proliferation induced by ex vivo TCR stimulation. Furthermore, deletion of FADD in preactivated mature T cells using retrovirus-Cre resulted in no proliferation. These results demonstrate that FADD plays a dispensable role during thymocyte development, but is essential in maintaining peripheral T cell homeostasis and regulating both apoptotic and proliferation signals.
Figures





Similar articles
-
FADD deficiency impairs early hematopoiesis in the bone marrow.J Immunol. 2011 Jan 1;186(1):203-13. doi: 10.4049/jimmunol.1000648. Epub 2010 Nov 29. J Immunol. 2011. PMID: 21115735 Free PMC article.
-
Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1.Nature. 1998 Mar 19;392(6673):296-300. doi: 10.1038/32681. Nature. 1998. PMID: 9521326
-
The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells.J Immunol. 2006 Jun 1;176(11):6852-61. doi: 10.4049/jimmunol.176.11.6852. J Immunol. 2006. PMID: 16709845 Free PMC article.
-
Molecular requirements for lineage commitment in the thymus--antibody-mediated receptor engagements reveal a central role for lck in lineage decisions.Immunol Rev. 1998 Oct;165:181-94. doi: 10.1111/j.1600-065x.1998.tb01239.x. Immunol Rev. 1998. PMID: 9850861 Review.
-
T cell immune reconstitution following lymphodepletion.Semin Immunol. 2007 Oct;19(5):318-30. doi: 10.1016/j.smim.2007.10.004. Epub 2007 Nov 19. Semin Immunol. 2007. PMID: 18023361 Free PMC article. Review.
Cited by
-
Lack of FADD in Tie-2 expressing cells causes RIPK3-mediated embryonic lethality.Cell Death Dis. 2016 Sep 1;7(9):e2351. doi: 10.1038/cddis.2016.251. Cell Death Dis. 2016. PMID: 27584790 Free PMC article. No abstract available.
-
Death receptor signal transducers: nodes of coordination in immune signaling networks.Nat Immunol. 2009 Apr;10(4):348-55. doi: 10.1038/ni.1714. Epub 2009 Mar 19. Nat Immunol. 2009. PMID: 19295631 Review.
-
Excessive apoptosis of Rip1-deficient T cells leads to premature aging.EMBO Rep. 2023 Dec 6;24(12):e57925. doi: 10.15252/embr.202357925. Epub 2023 Nov 15. EMBO Rep. 2023. PMID: 37965894 Free PMC article.
-
A novel genotyping strategy based on allele-specific inverse PCR for rapid and reliable identification of conditional FADD knockout mice.Mol Biotechnol. 2008 Feb;38(2):129-35. doi: 10.1007/s12033-007-9002-y. Epub 2007 Sep 15. Mol Biotechnol. 2008. PMID: 18219592
-
A novel function of RIP1 in postnatal development and immune homeostasis by protecting against RIP3-dependent necroptosis and FADD-mediated apoptosis.Front Cell Dev Biol. 2015 Feb 25;3:12. doi: 10.3389/fcell.2015.00012. eCollection 2015. Front Cell Dev Biol. 2015. PMID: 25767797 Free PMC article.
References
-
- von Boehmer H. Positive selection of lymphocytes. Cell. 1994;76:219. - PubMed
-
- Rathmell JC, Thompson CB. Pathways of apoptosis in lymphocyte development, homeostasis, and disease. Cell. 2002;109(Suppl):S97. - PubMed
-
- Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221. - PubMed
-
- Haks MC, Oosterwegel MA, Blom B, Spits H, Kruisbeek AM. Cell-fate decisions in early T cell development: regulation by cytokine receptors and the pre-TCR. Seminars in Immunology. 1999;11:23. - PubMed
-
- Strasser A, Bouillet P. The control of apoptosis in lymphocyte selection. Immunol Rev. 2003;193:82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

