Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene
- PMID: 16118273
- PMCID: PMC1200271
- DOI: 10.1073/pnas.0503264102
Variable sensitivity to noxious heat is mediated by differential expression of the CGRP gene
Abstract
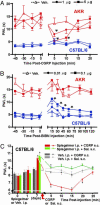
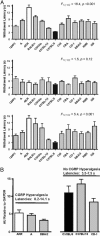
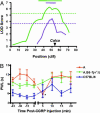
Heat sensitivity shows considerable functional variability in humans and laboratory animals, and is fundamental to inflammatory and possibly neuropathic pain. In the mouse, at least, much of this variability is genetic because inbred strains differ robustly in their behavioral sensitivity to noxious heat. These strain differences are shown here to reflect differential responsiveness of primary afferent thermal nociceptors to heat stimuli. We further present convergent behavioral and electrophysiological evidence that the variable responses to noxious heat are due to strain-dependence of CGRP expression and sensitivity. Strain differences in behavioral response to noxious heat could be abolished by peripheral injection of CGRP, blockade of cutaneous and spinal CGRP receptors, or long-term inactivation of CGRP with a CGRP-binding Spiegelmer. Linkage mapping supports the contention that the genetic variant determining variable heat pain sensitivity across mouse strains affects the expression of the Calca gene that codes for CGRPalpha.
Figures

References
-
- Mogil, J. S., Wilson, S. G., Bon, K., Lee, S. E., Chung, K., Raber, P., Pieper, J. O., Hain, H. S., Belknap, J. K., Hubert, L., et al. (1999) Pain 80, 67-82. - PubMed
-
- Mogil, J. S., Wilson, S. G., Bon, K., Lee, S. E., Chung, K., Raber, P., Pieper, J. O., Hain, H. S., Belknap, J. K., Hubert, L., et al. (1999) Pain 80, 83-93. - PubMed
-
- Bernardini, N., Neuhuber, W., Reeh, P. W. & Sauer, S. K. (2004) Neuroscience 126, 585-590. - PubMed
-
- Richardson, J. D. & Vasko, M. R. (2002) J. Pharmacol. Exp. Ther. 302, 839-845. - PubMed
-
- Sun, R.-Q., Tu, Y.-J., Lawand, N. B., Yan, J.-Y., Lin, Q. & Willis, W. D. (2004) J. Neurophysiol. 92, 2859-2866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

