Effects of the tumor inhibitory triterpenoid avicin G on cell integrity, cytokinesis, and protein ubiquitination in fission yeast
- PMID: 16118282
- PMCID: PMC1200287
- DOI: 10.1073/pnas.0505758102
Effects of the tumor inhibitory triterpenoid avicin G on cell integrity, cytokinesis, and protein ubiquitination in fission yeast
Abstract
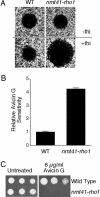
Avicins comprise a class of triterpenoid compounds that exhibit tumor inhibitory activity. Here we show that avicin G is inhibitory to growth of the fission yeast Schizosaccharomyces pombe. S. pombe cells treated with a lethal concentration of avicin G (20 microM) exhibited a shrunken morphology, indicating that avicin G adversely affects cell integrity. Cells treated with a sublethal concentration of avicin G (6.5 microM) exhibited a strong cytokinesis-defective phenotype (multiseptated cells), as well as cell morphology defects. These phenotypes bear resemblance to those resulting from loss of Rho1 GTPase function in S. pombe. Indeed, Rho1-deficient S. pombe cells were strongly hypersensitive to avicin G, suggesting that the compound may perturb Rho1-dependent processes. Consistent with previously observed effects in human Jurkat T cells, avicin G treatment resulted in hyperaccumulation of ubiquitinated proteins in S. pombe cells. Interestingly, proteasome-defective S. pombe mutants were not markedly hypersensitive to avicin G, whereas an anaphase-promoting complex (mitotic ubiquitin ligase) mutant exhibited avicin G resistance, suggesting that the increase in levels of ubiquitinated proteins resulting from avicin G treatment may be due to increased protein ubiquitination, rather than inhibition of 26S proteasome activity. Mutants defective in the cAMP/PKA pathway also exhibited resistance to avicin G. Our results suggest that S. pombe will be a useful model organism for elucidating molecular targets of avicin G and serve as a guide to clinical application where dysfunctional aspects of Rho and/or ubiquitination function have been demonstrated as in cancer, fibrosis, and inflammation.
Figures





References
-
- Mujoo, K., Haridas, V., Hoffmann, J. J., Wachter, G. A., Hutter, L. K., Lu, Y., Blake, M. E., Jayatilake, G. S., Bailey, D., Mills, G. B. & Gutterman, J. U. (2001) Cancer Res. 61, 5486–5490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

