Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes
- PMID: 16118321
- PMCID: PMC1895246
- DOI: 10.1182/blood-2005-06-2204
Differential roles of microtubule assembly and sliding in proplatelet formation by megakaryocytes
Abstract
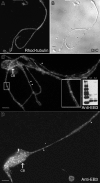
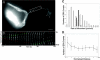
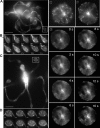
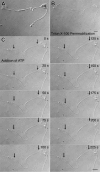
Megakaryocytes are terminally differentiated cells that, in their final hours, convert their cytoplasm into long, branched proplatelets, which remodel into blood platelets. Proplatelets elongate at an average rate of 0.85 microm/min in a microtubule-dependent process. Addition of rhodamine-tubulin to permeabilized proplatelets, immunofluorescence microscopy of the microtubule plus-end marker end-binding protein 3 (EB3), and fluorescence time-lapse microscopy of EB3-green fluorescent protein (GFP)-expressing megakaryocytes reveal that microtubules, organized as bipolar arrays, continuously polymerize throughout the proplatelet. In immature megakaryocytes lacking proplatelets, microtubule plus-ends initiate and grow by centrosomal nucleation at rates of 8.9 to 12.3 microm/min. In contrast, plus-end growth rates of microtubules within proplatelets are highly variable (1.5-23.5 microm/min) and are both slower and faster than those seen in immature cells. Despite the continuous assembly of microtubules, proplatelets continue to elongate when net microtubule assembly is arrested. One alternative mechanism for force generation is microtubule sliding. Triton X-100-permeabilized proplatelets containing dynein and its regulatory complex, dynactin, but not kinesin, elongate with the addition of adenosine triphosphate (ATP) at a rate of 0.65 microm/min. Retroviral expression in megakaryocytes of dynamitin (p50), which disrupts dynactin-dynein function, inhibits proplatelet elongation. We conclude that while continuous polymerization of microtubules is necessary to support the enlarging proplatelet mass, the sliding of overlapping microtubules is a vital component of proplatelet elongation.
Figures




References
-
- Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85: 402-413. - PubMed
-
- Cramer EM, Norol F, Guichard J, et al. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89: 2336-2346. - PubMed
-
- Leven RM, Yee MK. Megakaryocyte morphogenesis stimulated in vitro by whole or partially fractionated thrombocytopenic plasma: a model system for the study of platelet formation. Blood. 1987;69: 1046-1052. - PubMed
-
- Radley J, Rogerson J, Ellis S, Hasthorpe S. Megakaryocyte maturation in long-term marrow culture. Exp Hematol. 1991;19: 1075-1078. - PubMed
-
- Radley JM, Scurfield G. The mechanism of platelet release. Blood. 1980;56: 996-999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

