An array of microactuated microelectrodes for monitoring single-neuronal activity in rodents
- PMID: 16119243
- PMCID: PMC1635790
- DOI: 10.1109/TBME.2005.851478
An array of microactuated microelectrodes for monitoring single-neuronal activity in rodents
Abstract
Arrays of microelectrodes used for monitoring single- and multi-neuronal action potentials often fail to record from the same population of neurons over a period of time for several technical and biological reasons. We report here a novel Neural Probe chip with a 3-channel microactuated microelectrode array that will enable precise repositioning of the individual microelectrodes within the brain tissue after implantation. Thermal microactuators and associated microelectrodes in the Neural Probe chip are microfabricated using the Sandia's Ultraplanar Multi-level MEMS Technology (SUMMiTV) process, a 5-layer polysilicon micromachining technology of the Sandia National labs, Albuquerque, NM. The Neural Probe chip enables precise bi-directional positioning of the microelectrodes in the brain with a step resolution in the order of 8.8 microm. The thermal microactuators allow for a linear translation of the microelectrodes of up to 5 mm in either direction making it suitable for positioning microelectrodes in deep structures of a rodent brain. The overall translation in either direction was reduced to approximately 2 mm after insulation of the microelectrodes with epoxy for monitoring multi-unit activity. Single unit recordings were obtained from the somatosensory cortex of adult rats over a period of three days demonstrating the feasibility of this technology. Further optimization of the microelectrode insulation and chip packaging will be necessary before this technology can be validated in chronic experiments.
Figures





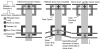








References
-
- BeMent SL, Wise KD, Anderson D, Najafi K, Drake KL. Solid state electrodes for multi channel multi-plexed intracortical neuronal recording. IEEE Trans Biomed Eng. 1986;BME-33:230–240. - PubMed
-
- Charles HK, Massey JT, Mountcastle VB. Polyimides as insulating layers for implantable electrodes. In: Mittal KL, editor. Polyimides. Vol. 2. New York: Plenum; 1984. pp. 1139–1155.
-
- Eichenbaum H, Kuperstein M. Extracellular neural recording with multichannel microelectrodes. J Electrophysiol Tech. 1986;13:189–209.
-
- Kuperstein M, Whittington DA. A practical 24 channel microelectrode for neural recording in vivo. IEEE Trans Biomed Eng. 1981;BME-28:288–293. - PubMed
-
- Pickard RS. A review of printed circuit microelectrodes and their production. J Neurosci Methods. 1979;1:301–319. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

